Recombinant Mouse IGFBP-3 protein (His Tag)
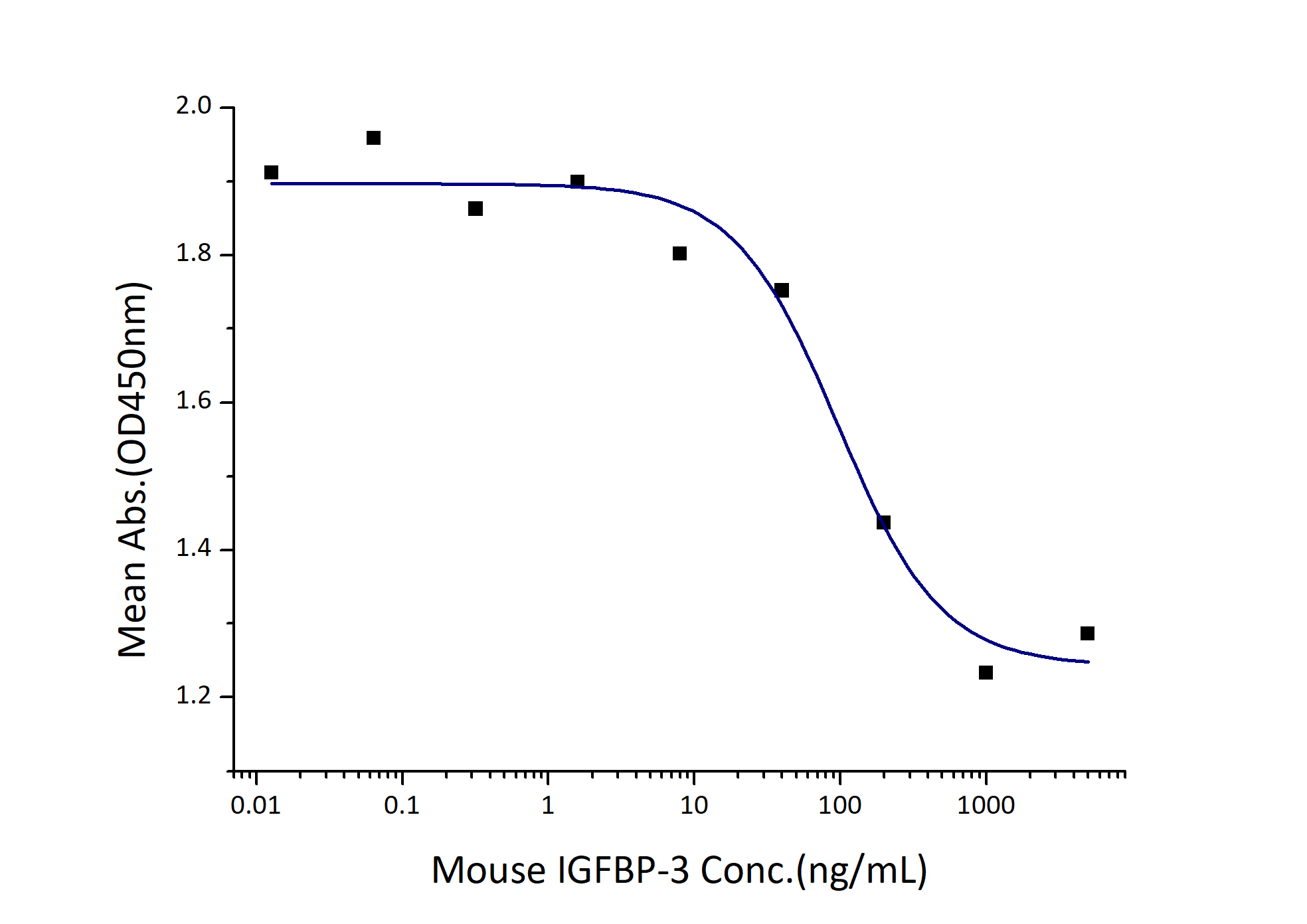
ED50
0.05-0.2 µg/mL
Species
Mouse
Purity
>95 %, SDS-PAGE
GeneID
16009
Accession
CAA57271.1
验证数据展示
Technical Specifications
| Purity | >95 %, SDS-PAGE |
| Endotoxin Level | <1.0 EU/μg protein, LAL method |
| Biological Activity |
Measured by its ability to inhibit the biological activity of IGF-I or IGFII on MCF-7 human breast cancer cells. The ED50 for this effect is 0.05-0.2 µg/mL in the presence of 30 ng/mL Recombinant human IGF-I. |
| Source | HEK293-derived Mouse IGFBP-3 protein Pro22-Gln291 (Accession# CAA57271.1, Arg250Gln, Gln259Arg, Ser260Gly, Arg271Pro) with a His tag at the C-terminus. |
| Predicted Molecular Mass | 30.4 kDa |
| SDS-PAGE | 45-55 kDa, reducing (R) conditions |
| Formulation | Lyophilized from sterile PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
Insulin-like growth factor binding protein-3 (IGFBP-3) contains a 27 amino acid putative signal sequence followed by a mature protein of 264 amino acids with 18 cysteine residues clustered near the N- and C-terminus. Accordingly, expression of the cloned IGFBP-3 cDNA in mammalian tissue culture cells results in secretion of the protein into the culture medium. IGFBP-3 shares high homology (33% amino acid identity) including conservation of all 18 cysteine residues with a smaller human IGF-binding protein (BP-28) identified in amniotic fluid. IGFBP-3 has one or more glycosylation sites with a protein core of ∼30 kDa. Western blots revealed that the 39-45 kDa IGFBP-3 fragment is a glycoprotein.
References:
1.Miljuš, G., Malenković, V., & Nedić, O. (2013). Metallomics,5(3), 251–258. 2. Renes, J. S., van Doorn, J., & Hokken-Koelega, A. C. (2014). J Clin Endocrinol Metab, 99(10):E1988-E1996. 3.Jia Y, Lee KW, Swerdloff R, et al. (2010). J Biol Chem, 285(3), 1726–1732.