验证数据展示
经过测试的应用
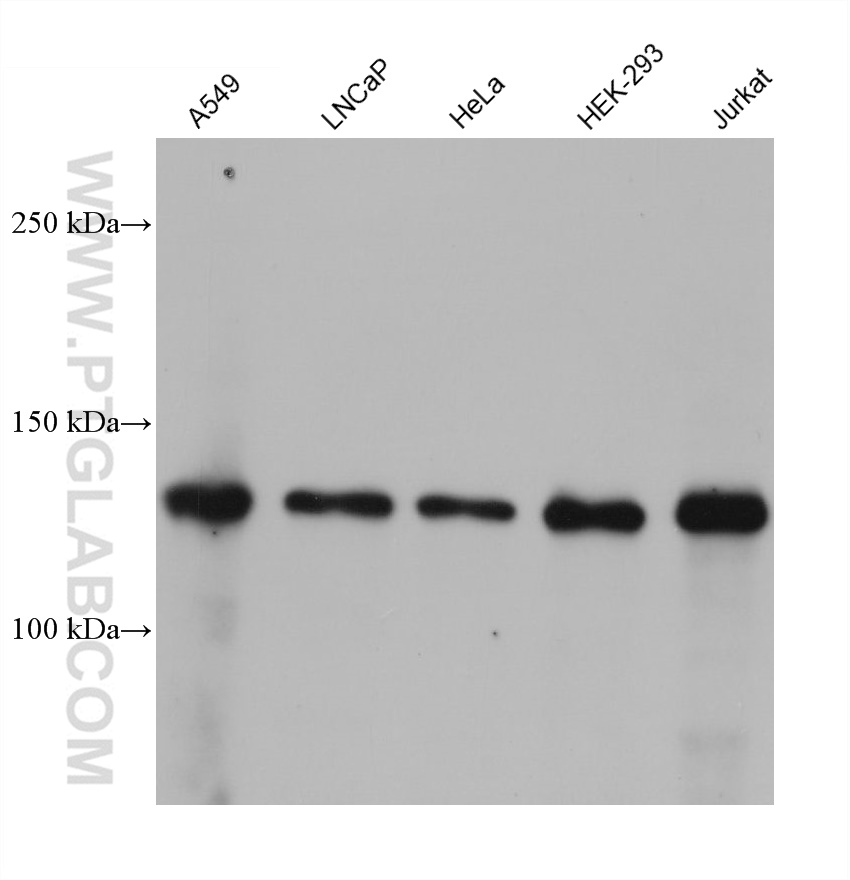
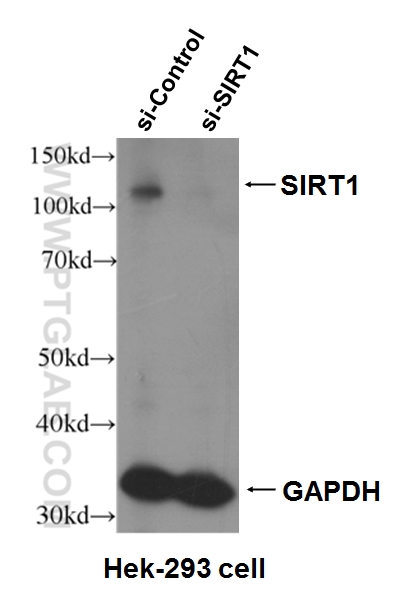
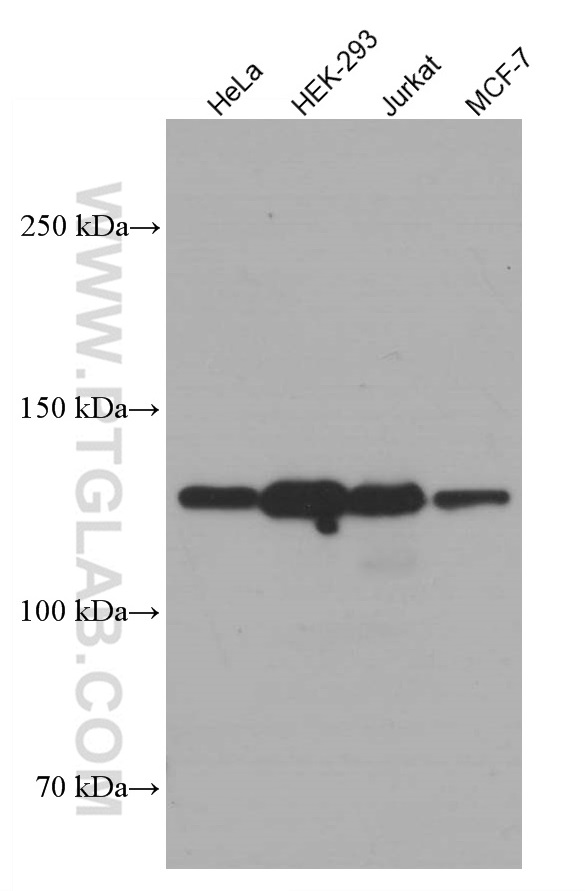
| Positive WB detected in | A549 cells, HeLa cells, Hek-293 cells, LNCaP cells, Jurkat cells, MCF-7 cells |
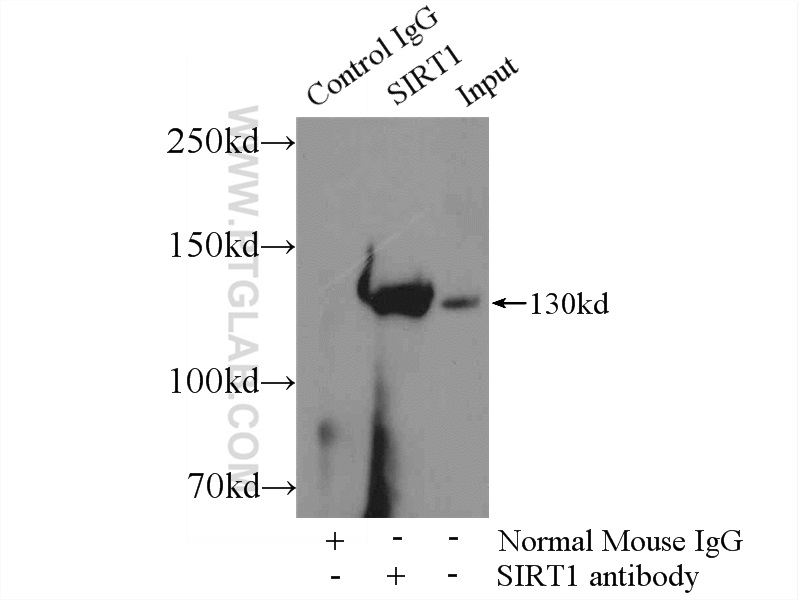
| Positive IP detected in | HeLa cells |
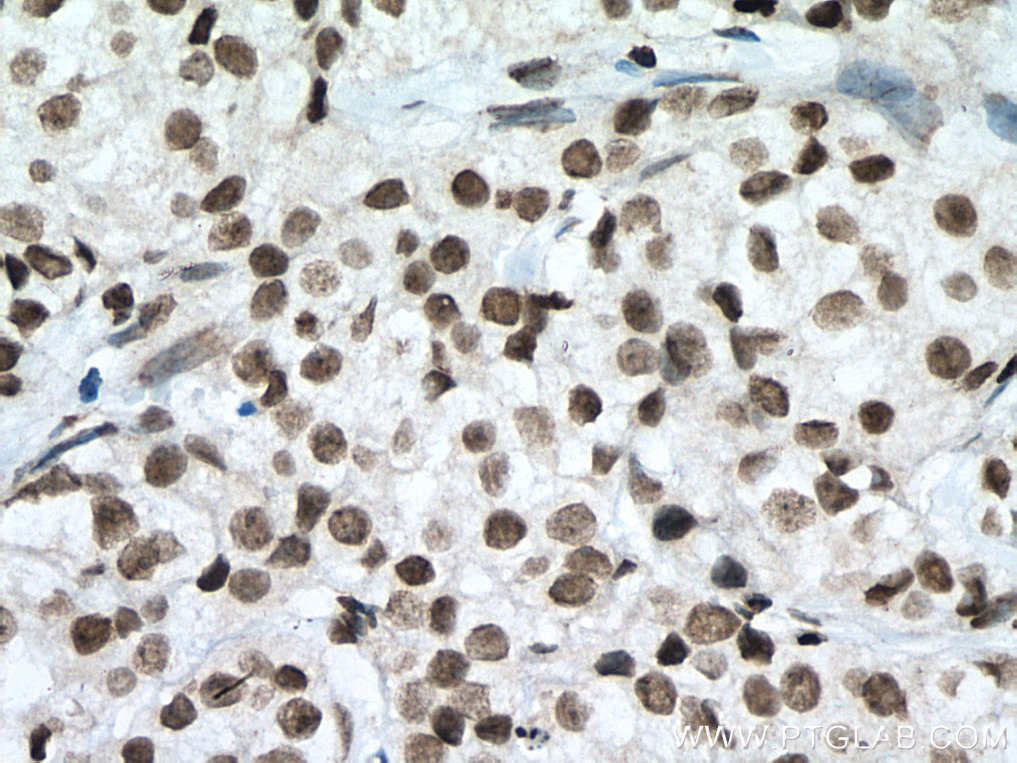
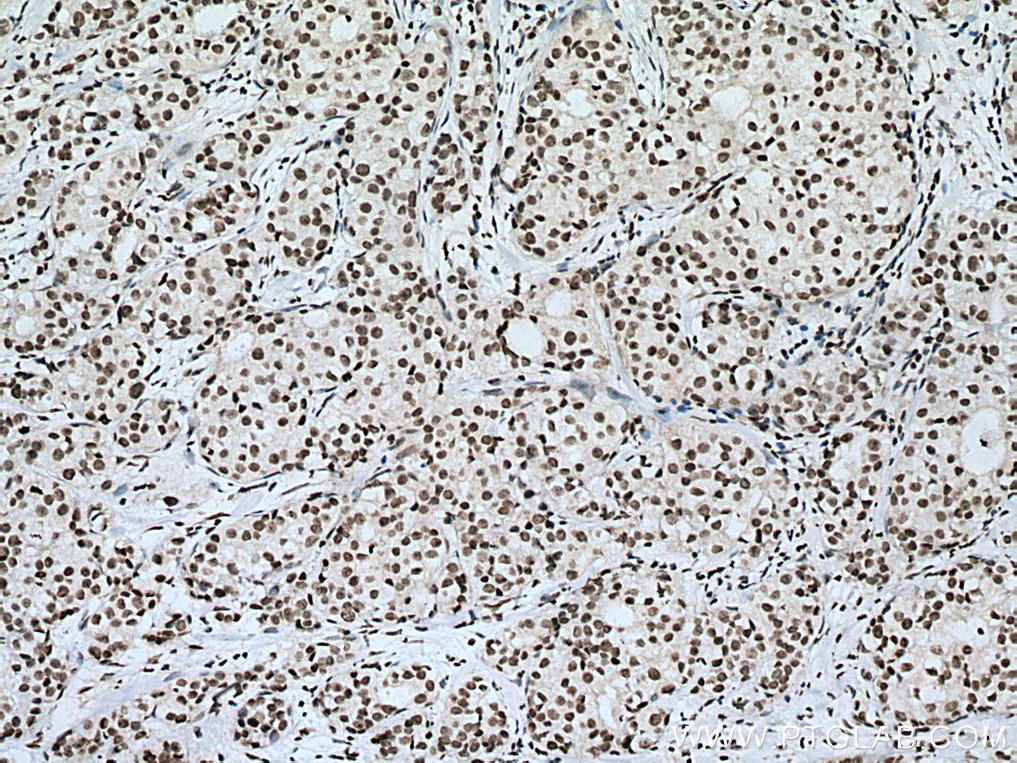
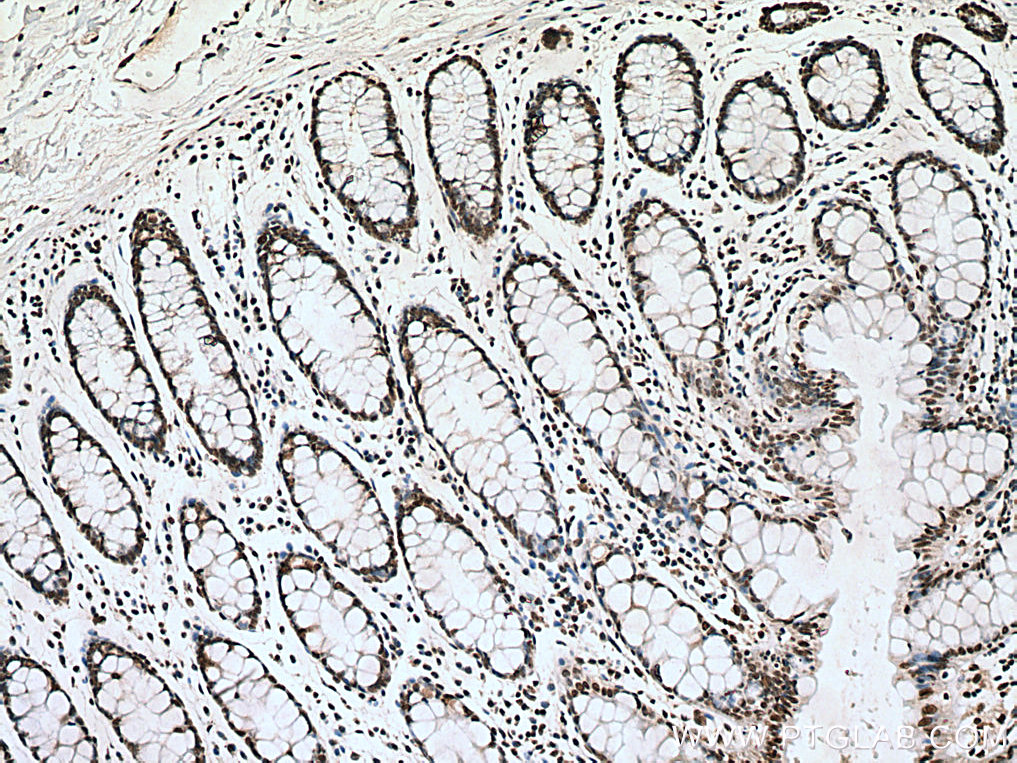
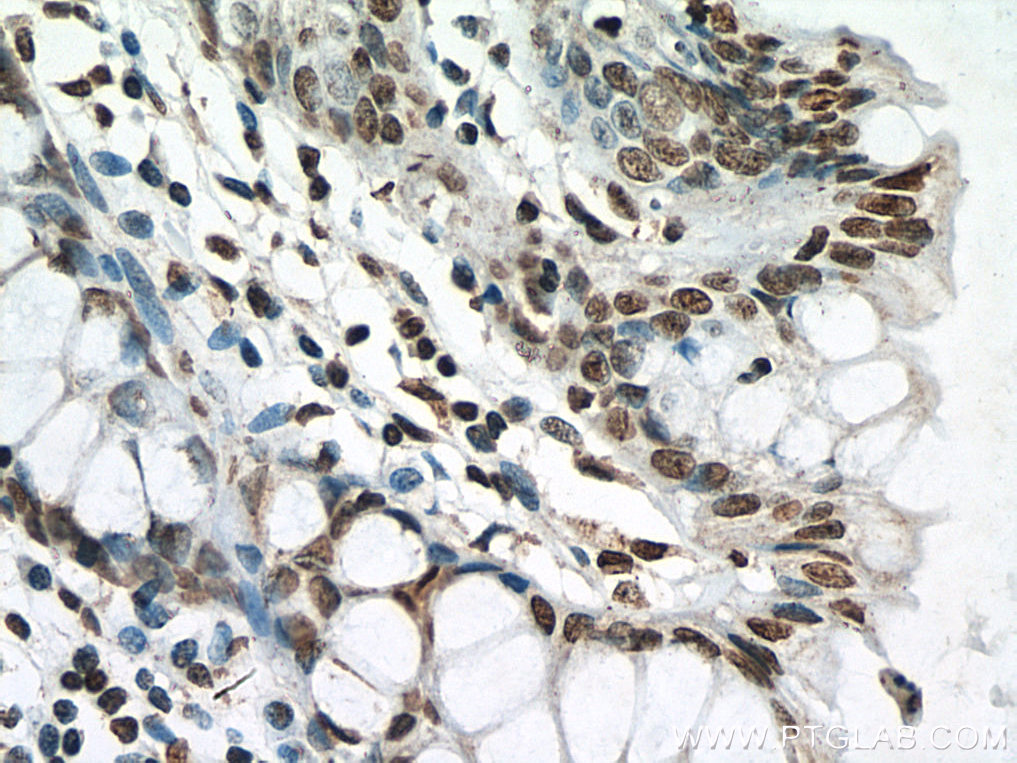
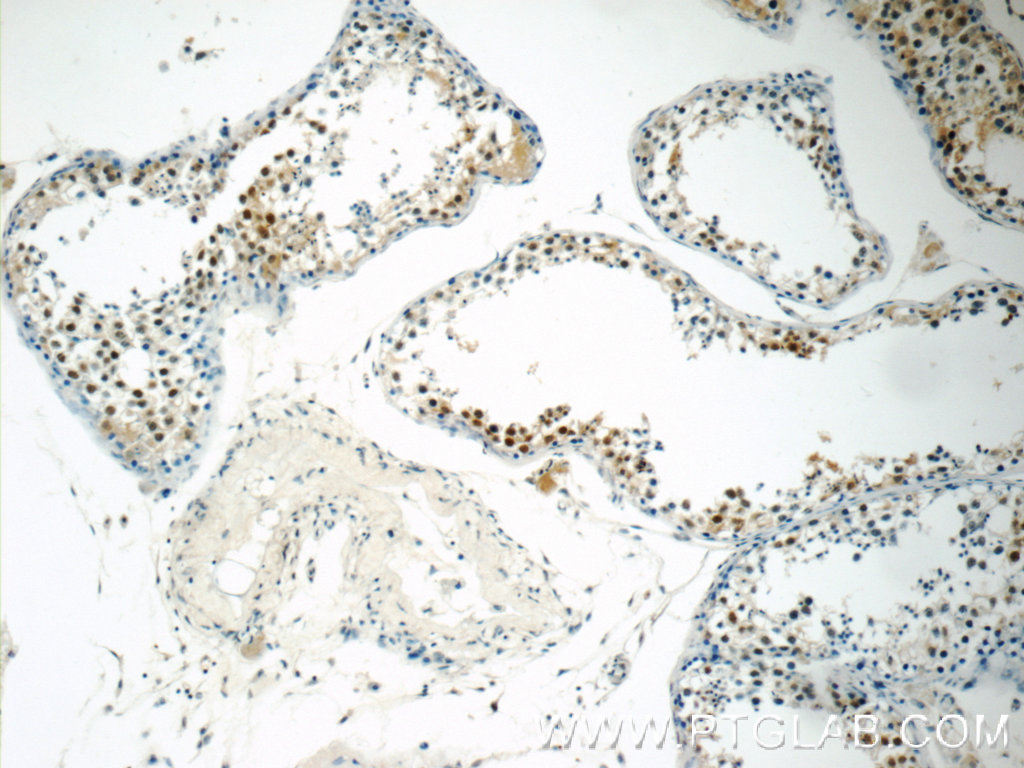
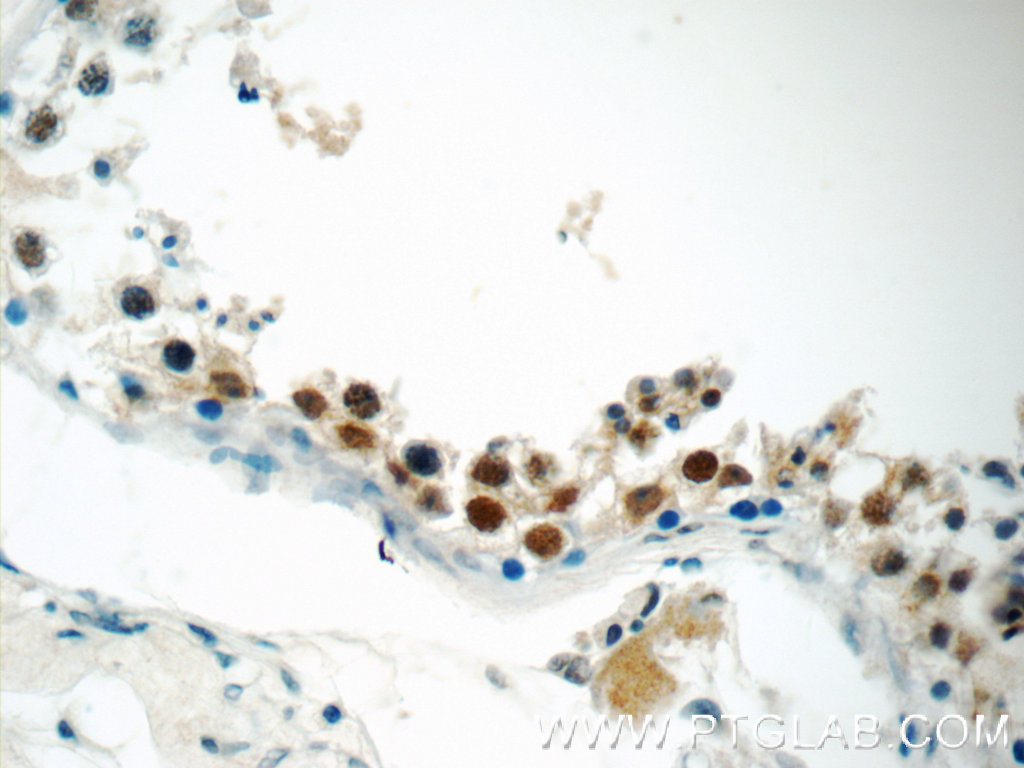
| Positive IHC detected in | human breast cancer tissue, human testis tissue, human colon tissue Note: suggested antigen retrieval with TE buffer pH 9.0; (*) Alternatively, antigen retrieval may be performed with citrate buffer pH 6.0 |
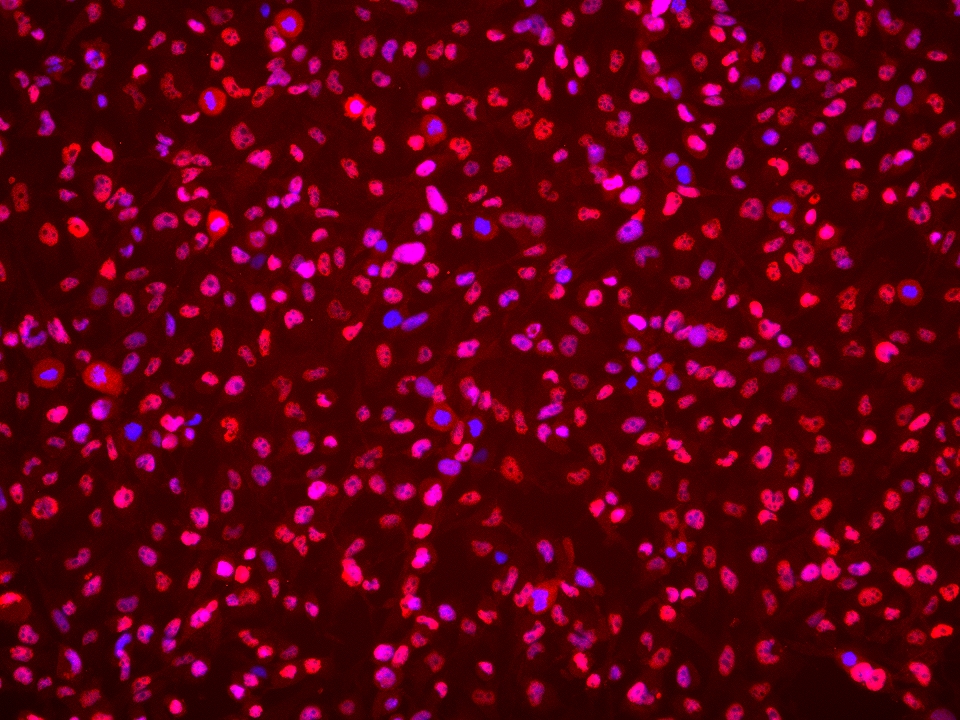
| Positive IF/ICC detected in | BT-549 cells |
推荐稀释比
| Application | Dilution |
|---|---|
| Western Blot (WB) | WB : 1:5000-1:50000 |
| Immunoprecipitation (IP) | IP : 0.5-4.0 ug for 1.0-3.0 mg of total protein lysate |
| Immunohistochemistry (IHC) | IHC : 1:250-1:1000 |
| Immunofluorescence (IF)/ICC | IF/ICC : 1:1000-1:4000 |
| It is recommended that this reagent should be titrated in each testing system to obtain optimal results. | |
| Sample-dependent, Check data in validation data gallery. | |
产品信息
60303-1-Ig targets SIRT1 in WB, IHC, IF/ICC, IP, CoIP, ELISA applications and shows reactivity with human samples.
| Tested Applications | WB, IHC, IF/ICC, IP, ELISA Application Description |
| Cited Applications | WB, IHC, IF, CoIP, ELISA |
| Tested Reactivity | human |
| Cited Reactivity | human, pig, rabbit |
| Immunogen | SIRT1 fusion protein Ag17677 种属同源性预测 |
| Host / Isotype | Mouse / IgG2b |
| Class | Monoclonal |
| Type | Antibody |
| Full Name | sirtuin (silent mating type information regulation 2 homolog) 1 (S. cerevisiae) |
| Synonyms | NAD-dependent protein deacetylase sirtuin-1, hSIRT1, hSIR2, EC:2.3.1.286, EC:2.3.1.- |
| Calculated Molecular Weight | 747 aa, 82 kDa |
| Observed Molecular Weight | 110-130 kDa |
| GenBank Accession Number | BC012499 |
| Gene Symbol | SIRT1 |
| Gene ID (NCBI) | 23411 |
| RRID | AB_2881417 |
| Conjugate | Unconjugated |
| Form | Liquid |
| Purification Method | Protein A purification |
| UNIPROT ID | Q96EB6 |
| Storage Buffer | PBS with 0.02% sodium azide and 50% glycerol pH 7.3. |
| Storage Conditions | Store at -20°C. Stable for one year after shipment. Aliquoting is unnecessary for -20oC storage. |
背景介绍
SIRT1, also named as SIR2L1, contains a deacetylase sirtuin-type domain and belongs to the sirtuin family. The post-translation modified SIRT1 is a 110-130 kDa protein, which contains one deacetylase sirtuin-type domain. The 75-80 kDa SIRT1 fragment was detected to lack the carboxy-terminus (PMID:21305533). SIRT1 exists a 57-61 kDa isoform. SIRT1 may be found in nucleolus, nuclear euchromatin, heterochromatin and inner membrane. It can shuttles between nucleus and cytoplasm. SIRT1 regulates processes such as apoptosis and muscle differentiation by deacetylating key proteins. SIRT1 in particular initiates several signaling events relevant to cardioprotection, including: activation of endothelial nitric oxide synthase, INS receptor signaling, and autophagy. In addition SIRT1 activation elicits resistance to oxidative stress via regulation of transcription factors and co-activators such as FOXO, Hif-2a, and NF-kB. SIRT1 regulates the p53-dependent DNA damage response pathway by binding to and deacetylating p53, specifically at Lysine 382.
实验方案
| Product Specific Protocols | |
|---|---|
| WB protocol for SIRT1 antibody 60303-1-Ig | Download protocol |
| IHC protocol for SIRT1 antibody 60303-1-Ig | Download protocol |
| IF protocol for SIRT1 antibody 60303-1-Ig | Download protocol |
| IP protocol for SIRT1 antibody 60303-1-Ig | Download protocol |
| Standard Protocols | |
|---|---|
| Click here to view our Standard Protocols |
发表文章
| Species | Application | Title |
|---|---|---|
J Pineal Res Melatonin Protects Mouse Testes from Palmitic Acid-Induced Lipotoxicity by Attenuating Oxidative Stress and DNA Damage in a SIRT1-Dependent Manner. | ||
Free Radic Biol Med Menaquinone-4 prevents medication-related osteonecrosis of the jaw through the SIRT1 signaling-mediated inhibition of cellular metabolic stresses-induced osteoblast apoptosis | ||
Int J Mol Sci Exposure to Bisphenol A Caused Hepatoxicity and Intestinal Flora Disorder in Rats. | ||
Int J Mol Sci COX-2/sEH Dual Inhibitor Alleviates Hepatocyte Senescence in NAFLD Mice by Restoring Autophagy through Sirt1/PI3K/AKT/mTOR. | ||
J Cell Physiol Y-box binding protein 1 influences zygotic genome activation by regulating N6-methyladenosine in porcine embryos | ||
J Cell Physiol Acetyl-CoA synthases are essential for maintaining histone acetylation under metabolic stress during zygotic genome activation in pigs. |