Secondary Antibody Selection
How to choose the best secondary antibody for your application
Secondary antibodies are critical for successful experiments involving antibodies. They provide signal detection and amplification along with extending the utility of an antibody through conjugation to proteins. There are several different types of secondary antibodies and selecting the best one can be a challenge. The article below will help you make the best choice.
Browse all secondary antibodies |
What is the host species of your primary antibody?
The secondary antibody must be raised against the host species of the primary antibody. For example, if the primary antibody was generated in rabbit, the secondary needs to be anti-rabbit
 |
Figure 1. Most common host species of primary antibody.
Is your primary antibody polyclonal or monoclonal?
Polyclonal antibody
A polyclonal antibody contains a mixture of several isotypes of immunoglobulin G (e.g. IgG1, IgG2a). Therefore, to maximize detection of the target, it is best to use a secondary antibody that recognizes all isotypes. These are any secondary antibodies which are labeled “IgG H+L” (H and L represent heavy and light chains of IgG, respectively). This designation means the secondary host species was immunized with a pool of IgG’s from another species allowing the purified secondary antibody to recognize all forms.
Monoclonal antibody
A monoclonal antibody contains a single isotype of immunoglobulin. Therefore, it is critical to use a secondary that specifically recognizes that isotype. The isotype of the primary antibody should be indicated on the product’s datasheet.
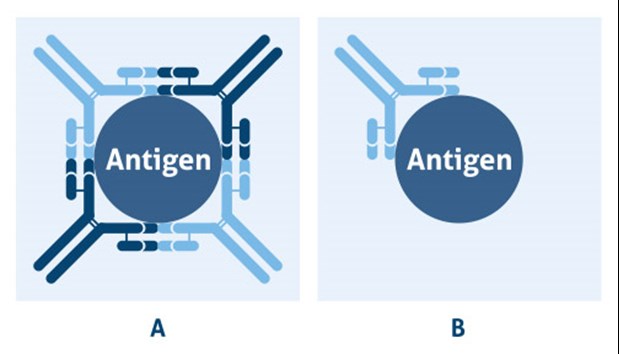 |
Figure 2. Categories of antibodies: A) polyclonal antibodies are binding to the same antigen, but different epitopes and B) monoclonal antibodies are binding to the same epitope on a target antigen.
Please note: If the primary antibody is a fragment (not the whole antibody), the secondary antibody should also be fragment-specific to reduce noise signal (e.g., F(ab’)₂ fragment secondary antibodies penetrate tissue easier in IHC).
What is your method of signal detection?
Secondary antibodies can be conjugated to a wide range of chemicals, including enzymes (horseradish peroxidase (HRP) or alkaline phosphatase (AP)) and fluorophores (e.g. fluorescein (FITC) or Cyanine (Cy3)).
Other considerations
Purification:
A common purification technique for secondary antibodies is High-Affinity Purification, resulting in reduced non-specific binding. In addition, secondary antibodies can go through an additional purification step to reduce potential cross-reactions with other species called cross-adsorption. In this process, the secondary antibody solution is passed over different columns containing sera proteins of different species to filter out the non-specific secondary antibody (e.g., for multi-staining).
Western Blotting after Immunoprecipitation:
When using Western blot to detect a protein in immunoprecipitated samples, extra care should be given to the choice of antibodies. Immunoprecipitated samples contain the pull-down antibody’s heavy and light chains. These chains show up at 100 kDa and 50 kDa. If the secondary antibody recognizes the pull-down antibody and the Western blot’s primary antibody these bands will show up at a high intensity and may overshadow the target protein signal. It is best to use a different species for the Western Blot’s primary antibody than the pull-down antibody. If this is not possible it is recommended to use a secondary that recognizes the specific chain furthest away from your target protein or to use a secondary antibody that prefers the native over denatured forms.


