1. How long will my antibody last if it is properly stored?
Our antibodies are guaranteed for 1 year after purchase. However, our internal studies show that the antibody will remain functional for a minimum of 5 years if properly stored.
2. How should I store my antibodies? Do I need to store it in aliquots?
Please store the antibody in its original tube at -20°C. Our antibodies are stored in 50% glycerol, which prevents them from freezing. Aliquoting is unnecessary for -20°C storage.
3. Could you send me detailed information for this antibody?
Yes! Feel free to email us with the product number in the subject line. Alternatively, when you are on a specific product page, you can find a printable datasheet by clicking the Print Icon below the product number at the top of the page (Figure 1).
4. What is the concentration of my antibody?
The concentration of a given antibody is found on the product datasheet and on the product vial tube itself. In the event that the concentration displayed on the vial tube is different to that shown on the product datasheet, please refer to the vial tube concentration.
5. Can you provide me with the immunogen sequence of this antibody?
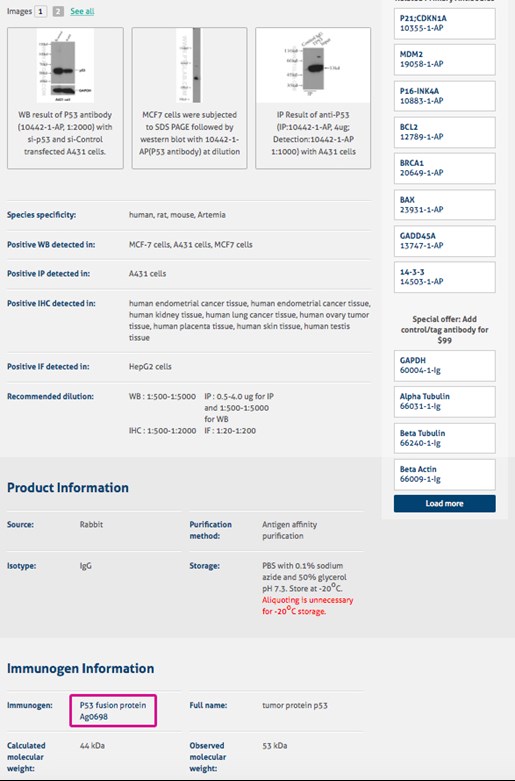
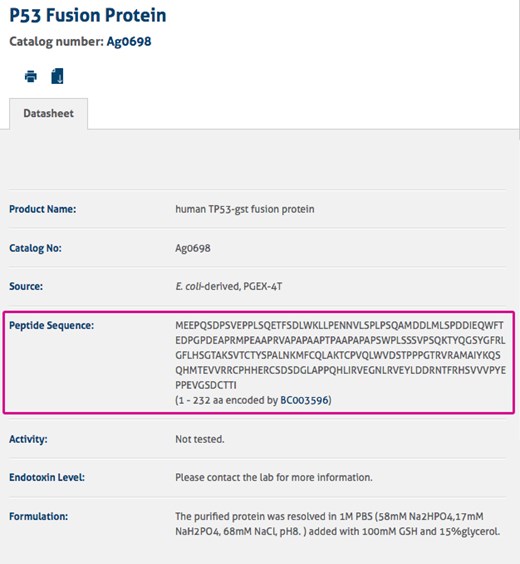
On the antibody product page, please click the link beside ‘Immunogen’ under the ‘Immunogen Information’ section (Figure 2) to view the fusion protein with the amino acid sequence (Figure 3). Should you require more detailed information, please speak to a member of our staff via live chat.
6. Is there a blocking peptide available for my antibody?
Yes, we offer blocking proteins for your experiments. A link to these products can be found beside ‘Immunogen’ under the ‘Immunogen Information’ section of the antibody product page.
7. Has my antibody been tested on any species other than human?
We usually test our antibodies on human and mouse cells or tissues. However, our guarantee covers the antibody in any species.
8. Which applications can my antibody be used in?
All currently tested applications are listed on each product page. Should you require more details or information regarding an application other than those listed, please contact our team.
9. Does my antibody cross-react with other species?
This depends on the extent of protein sequence similarity between the immunogen and the potential cross-reactive protein sequence. A pair-wise sequence alignment can be performed online through the NCBI-BLAST website.
10. Has the antibody already been published?
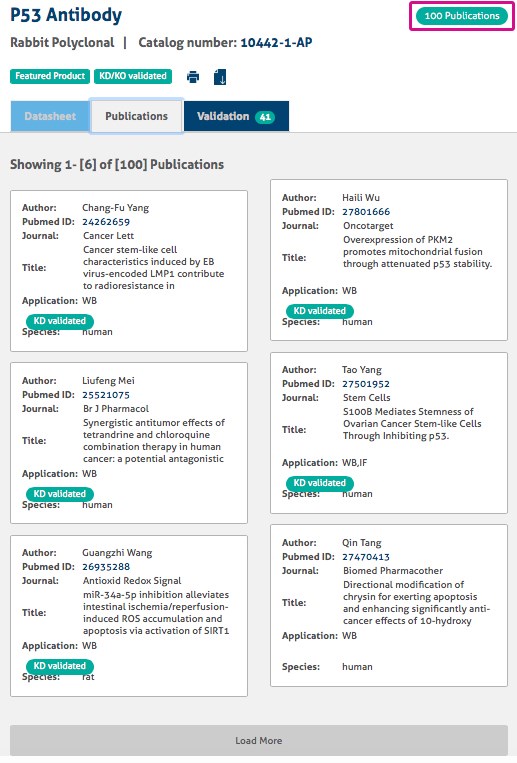
Each product page shows the number of publications and has links to the publication if the antibody has already been cited (Figure 4).
11. How do I choose a suitable secondary antibody?
The choice of appropriate secondary antibody is determined by the specifications of the primary antibody and the application (i.e., species, subclass, and fragment type).
Guiding principles for choosing a suitable secondary antibody:
- The secondary antibody must be raised against the host species of the primary antibody (e.g., anti-mouse or rabbit)
- The secondary antibody must be specific to the isotype class of the primary antibody (e.g., IgG or IgM)
- If the primary antibody is a fragment (e.g., F(ab’)2), the secondary antibody should target that specific fragment to reduce background noise
Proteintech offers a variety of secondary antibodies suitable for Western Blotting, ELISA, cellular imaging, and flow cytometry. View our secondary antibodies catalog for more detailed information.
12. How should I choose an isotype control?
Isotype controls are used to determine non-specific interactions in a given sample such as Fc receptor binding. Most isotype controls are monoclonal and most are not suitable for use with polyclonal antibodies as they contain more than one IgG class. The isotype control antibody should match the primary antibody regarding host species, isotype, and conjugation. Lastly, the sample incubated with the isotype control antibody and the sample incubated with the primary antibody should always be run in parallel.
13. How can I choose a positive and a negative control?
A sample without a primary antibody can be used as a negative control to distinguish specific and non-specific signal from the secondary antibody. Any products/stainings obtained with these control conditions can be attributed to non-specific (off-target) interactions. Furthermore, samples that have been genetically manipulated to alter the target’s levels (e.g., CRISPR) can be used as a negative control.
For a positive control, any tissues, cells, or lysates that have been used successfully in our validation data set or by our customers are a suitable control. Each antibody has its own PubMed publication record on our website. Additionally, the Uniprot, Omnigene, and GeneCards databases are great resources for finding tissues/cell lines with high expression of the target. The latest publications record in PubMed can also be a useful tool for detailed and the most up-to-date information regarding target research.
14. What is the clone number?
Each monoclonal antibody has a clone number that is specific for its single clone of hybridoma cells. The clone number can be found on the product page next to the catalog number (Figure 5).

15. Why is the predicted WB band size different from the actual one?
There may be several reasons for a difference in band size, such as multimers, gel migration, relative charges, splice variants, post-translational modification, or cleavage. For more information, please contact our technical support.
16. Should I perform an antigen retrieval step for my IHC experiment?
Yes, it is recommended to perform an antigen retrieval step since the fixation process during paraffinization cross-links proteins and may mask the epitopes, resulting in weak or false negative staining. However, this situation can be rectified with a heat-induced epitope retrieval (HIER) or proteolytic-induced epitope retrieval (PIER) step. The most suitable choice depends on the tissue type and primary antibody.
For more detailed information regarding antigen retrieval steps, please check our IHC guide and protocols page.
1. What method does Proteintech use for recombinant protein purification?
We usually add 6x His tags or GST tags to the N-terminus of proteins; these proteins are then purified by Ni2+ and glutathione affinity chromatography, respectively.
2. What storage buffers are used for your recombinant protein?
The His-tagged recombinant protein is usually stored in 1x PBS buffer (58mM Na2HPO4, 17mM NaH2PO4, 68mM NaCl, pH 7.4) containing 300mM imidazole.
The GST-tagged recombinant protein is usually stored in 1x PBS buffer (58mM Na2HPO4, 17mM NaH2PO4, 68mM NaCl, pH 7.4) containing 100mM glutathione (GSH).
1. What does the ELISA kit contain?
-
Antibody-coated 96-well microplate
-
Assay standard
-
Detection antibody
-
HRP-conjugated antibody
-
Sample diluent
-
Detection diluent
-
Wash buffer
-
Tetramethylbenzidine (TMB) substrate
-
Stop solution
-
Plate cover seals
2. Which ELISA type do you offer?
All Proteintech ELISA kits are two-site sandwich ELISAs.
3. Are the plates pre-coated?
Yes, all plates are pre-coated with an antibody that is specific for the target.
4. Do I have to run the ELISA standards and samples in duplicate/triplicate?
It is recommended to run the experiment in duplicate at a minimum. Triplicate would be ideal.
5. Is it possible to use the reagents of two different ELISA kits/lots?
No, different lot numbers cannot be mixed.
6. Are shorter or longer incubation times possible?
Incubation times should be followed exactly as stated in the manual.
7. How should I store the reagent?
Proteintech ELISA kits can be stored at 4C for 6 months after receipt.
8. Can I use a non-validated sample type with this ELISA kit?
Most of our ELISA kits are validated for cell culture supernatant, plasma, or serum.
9. How sensitive are the ELISA kits?
The ELISA sensitivity is highlighted in the data sheet for each kit.
10. Why does my standard shows very low or no color development?
There are multiple explanations: incorrect storage, incorrect incubation time, improper antibody, insufficient washing, etc. For more information, please contact technical support.
11. What are the reasons for high background?
Improper washing, contaminated reagents, samples, or wells. For more information, please contact technical support.
12. Is it possible to store samples after the collection?
Fresh samples are ideally recommended. If you need to keep the samples, you can store at 4 °C for short-term storage(<3 days) or at -20 °C/-80°C for long-term storage(up to 6 months). Please avoid freeze/thaw cycles.
1. How long will my custom production project take?
Custom production projects can typically take up to 6 months to complete, but due to the complexity of the process, we cannot always give a guarantee of an exact schedule. Please see below for the schedule of our 102-day immunization protocol, which demonstrates our commitment to making sure that you receive an antibody that works.
102-Day Immunization Protocol
| Day 0 Pre-Immune Bleed | Day 56 Test Bleed & ELISA | Day 88 Production Bleed |
| Day 1 Primary Injection | Day 60 Boost 3 | Day 102 Final Bleed |
| Day 28 Boost 1 | Day 74 Production Bleed | |
| Day 42 Boost 2 | Day 78 Boost 4 |
2. Can I express the fusion protein in my lab and send you that?
No, unfortunately we do not currently accept fusion proteins expressed by our customers.
3. What carrier proteins do you use?
KLH is the default carrier protein. This is linked to the peptide via a terminal cysteine (added by Proteintech®). If you have cysteine residue in the middle of your peptide then we can use MAP beads instead.
4. How many rabbits are immunized for each peptide service?
Each antigen will be injected into two rabbits.
5. Should I choose a full-length fusion protein or peptide antigen?
In our experience, full-length fusion protein antigens usually generate antibodies with higher titers and higher sensitivities. We recommend the full-length fusion protein procedure if your protein of interest has low sequence similarity with other proteins. However, if you need an antibody that is specific to a certain protein, the sequence of which is highly similar to other proteins, we strongly recommend using a peptide antigen. Other reasons you might choose a peptide antigen include:
1. Not having the cDNA for the protein of interest;
2. Expression of the full-length fusion protein has not been successful for some reason.
6. Is your custom antibody production service limited to human genes?
No, we carry out custom production of antibodies for proteins from any species, depending on antigenicity.
7. How much antiserum will I receive?
Each rabbit will yield approximately 12 ml of serum in each production bleed and 45–50 ml of serum from 90–100 ml of blood in the final bleed, totalling about 150 ml of antiserum from two rabbits.
8. How does Proteintech determine which rabbit to purify serum from?
We decide that based on the ELISA results with the test bleeds. If both rabbits give good results, we will purify 15 ml of serum from each rabbit. If one rabbit gives better results than the other, we will purify 30 ml of serum from this rabbit.
9. How much purified antibody can I get from the antiserum?
It depends on the concentration of the specific antibody in the serum provided. Usually we can get 2–10 ml of purified antibody from 15 ml of antiserum.
1. What is the range for EC50?
The EC50 is defined as the concentration at which the bioassay activity is 50% of the maximum response.
2. How do you convert EC50 to specific activity in units/mg?
Due to the variable nature of bioassays, the same preparation may not give the exact same EC50 value. Any variation in a bioassay condition such as cell densities or passage numbers may lead to variable results. To reflect this biological variability, Proteintech reports the typical EC50 range on the datasheet for a given protein. The formula for converting the EC50 in ng/ml to specific activity in units/mg is:

3. What are the advantages of Humankine products over E. coli, CHO, and insect cell expressed proteins?
Recombinant Humankine cytokines and growth factors are expressed in an optimized, proprietary human cell line of HEK293 origin. Proteins produced using our technology have native glycosylation, folding, dimerization, and post-translational processing, so their biological activity and stability are superior to proteins expressed in non-human systems.
4. How are Humankine products shipped?
Humankine products are shipped at room temperature (RT) as lyophilized and carrier-free proteins. Before opening, we recommend briefly centrifuging the vial and then proceeding to follow the product-specific instructions as described either on the datasheet and/or certificate of analysis to reconstitute.
5. How should I store Humankine products?
Proteins are stable for one year from the date of receipt if stored between (-20°C) and (-80°C). All products are lyophilized, except for Thrombin (HZ-3010).
6. In what applications can Humankine products be used?
Humankine products are broadly used for stem cell research and cell therapy. At the time of writing, Humankine products have been cited in more than 250 PubMed publications. See the specific product pages for more details.
7. What type of media does Proteintech use for Humankine production with our expression system? Does Proteintech use animal components such as FBS?
We use commercially available, chemically defined serum and xeno-free media. It is completely free of animal components.
8. What is the recommended concentration for the reconstitution step?
We recommend reconstituting our proteins at less than or equal to 1mg/mL (except Wnt3a).
9. Is your cell line available from ATCC?
Should you require more details or information about our cell line, please contact our team.
10. Does Proteintech manufacture a GMP-grade recombinant protein?
Please contact us for more information on GMP-grade proteins. GMP proteins can be produced on custom request.