Nano Secondaries®纳米二抗
纳米级大小精细成像
Nano Secondaries®是一种可以获得更高的分辨率、更低的背景和更清晰的图像的新型二抗。该二抗是与荧光染料结合的纳米抗体/VHHS,可识别一抗的Fab或Fc片段。
-
空间位阻小,分辨率极高
-
通过SRM, IF, WB, FC验证
-
极小的物种交叉反应性
-
快速染色:一步孵育
-
批间高度稳定,重复性高
Nano Secondaries® 纳米二抗产品列表
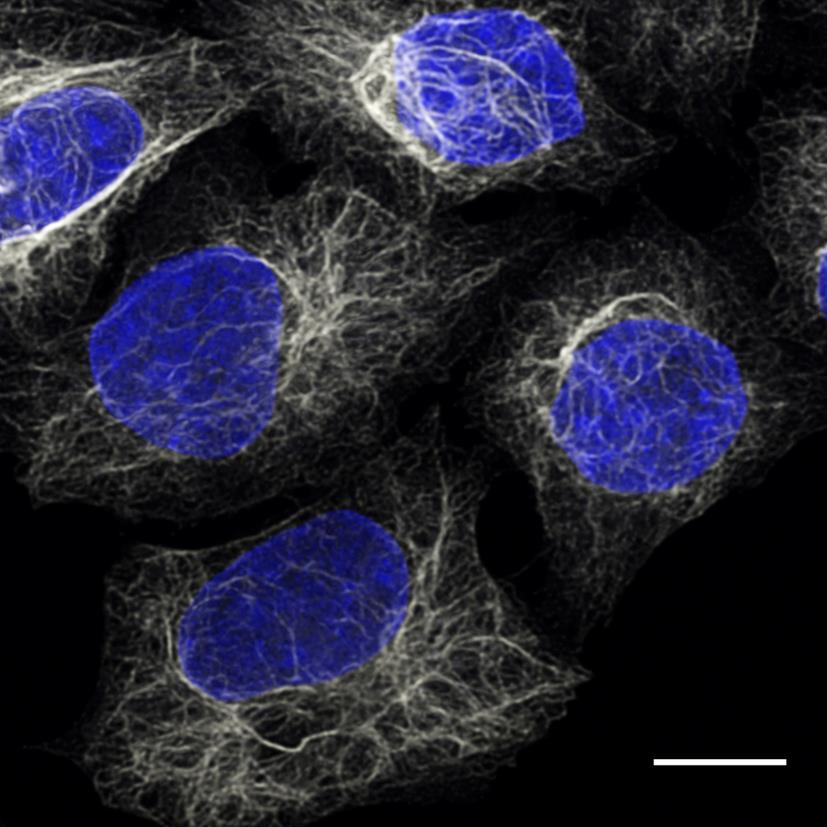
HeLa cells were immunostained with anti-Vimentin mouse IgG1 antibody and alpaca anti-mouse IgG1 VHH Alexa Fluor® 647 (grey). Nuclei were stained with DAPI, blue. Scale bar, 10 μm. Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
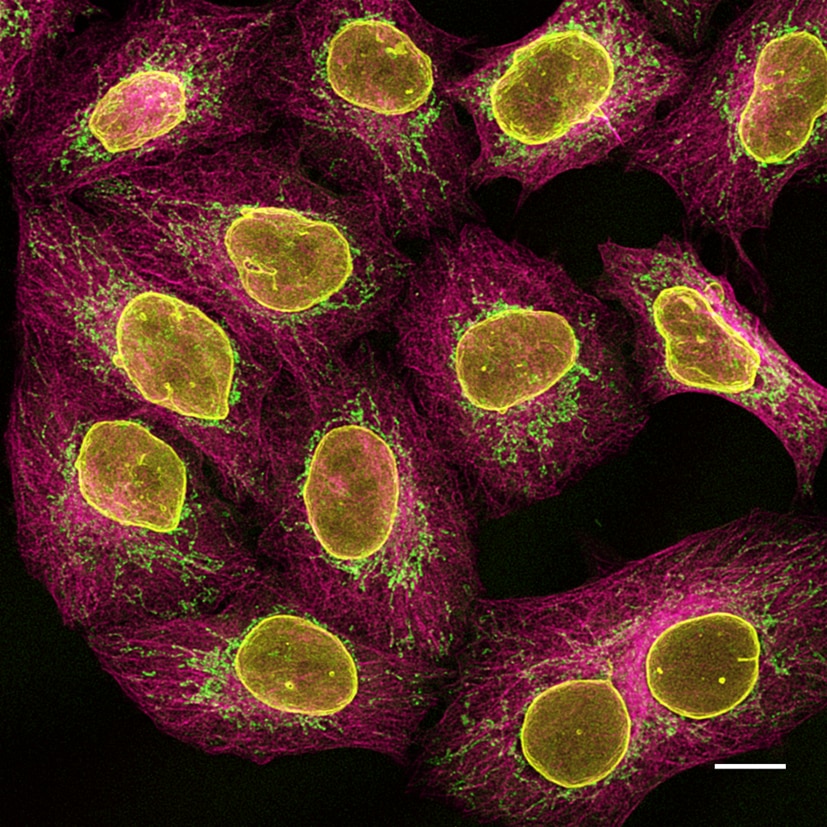
Multiplexed immunostaining of HeLa cells with two alpaca anti-mouse Nano-Secondaries and one anti-rabbit Nano-Secondary. Green: mouse IgG1 anti-COX4 + alpaca anti-mouse IgG1 VHH Alexa Fluor 488. Magenta: mouse IgG2b anti-Tubulin + alpaca anti-mouse IgG2b VHH Alexa Fluor 647. Yellow: rabbit anti-Lamin + alpaca anti-rabbit IgG VHH Alexa Fluor 568. Scale bar, 10 μm. Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
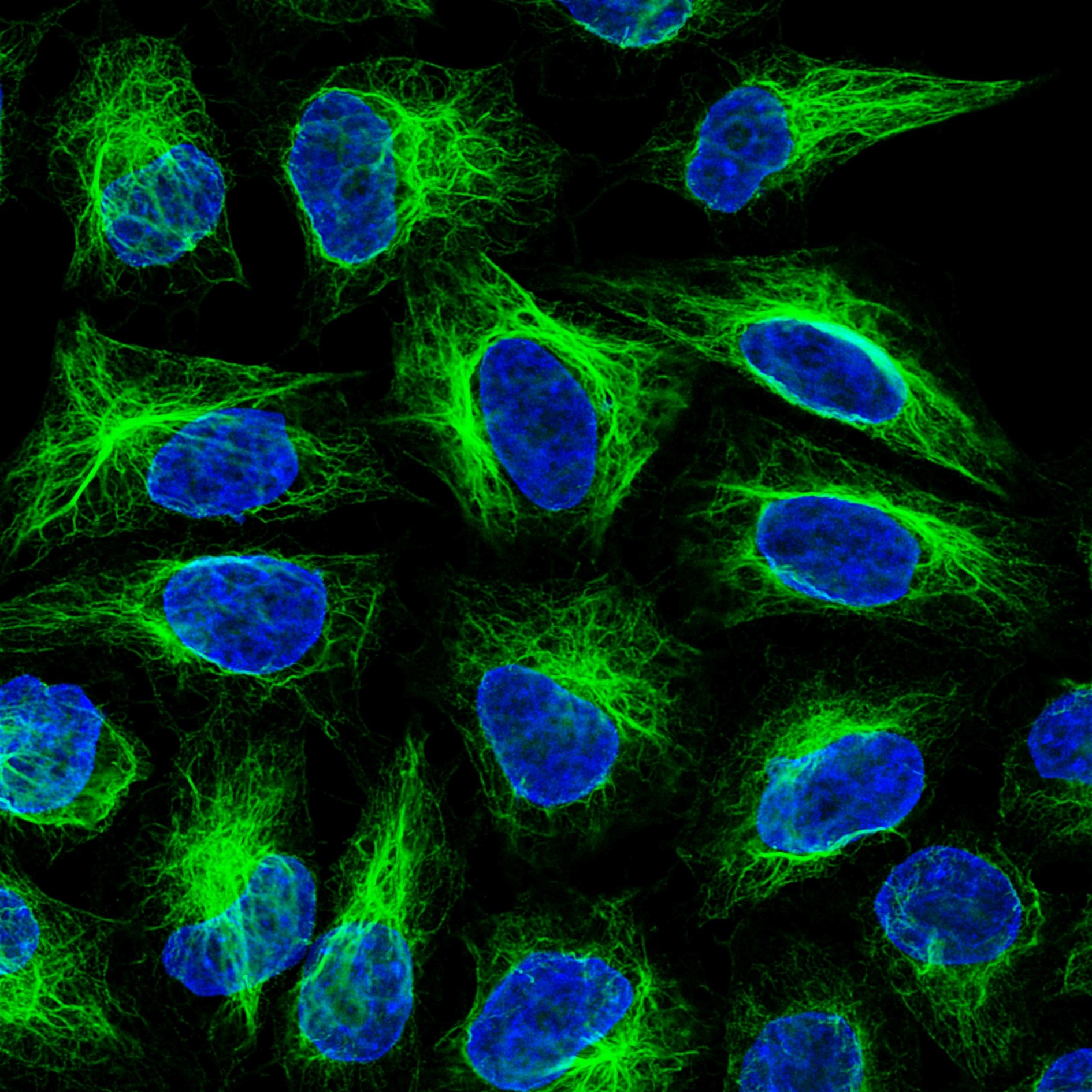
Immunofluorescence analysis of HeLa cells stained with mouse IgG1 anti-vimentin antibody and Nano-Secondary® alpaca anti-mouse IgG1, recombinant VHH, CoraLite® Plus 488 (smsG1CL488-1, green). Nuclei were stained with DAPI (blue). Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
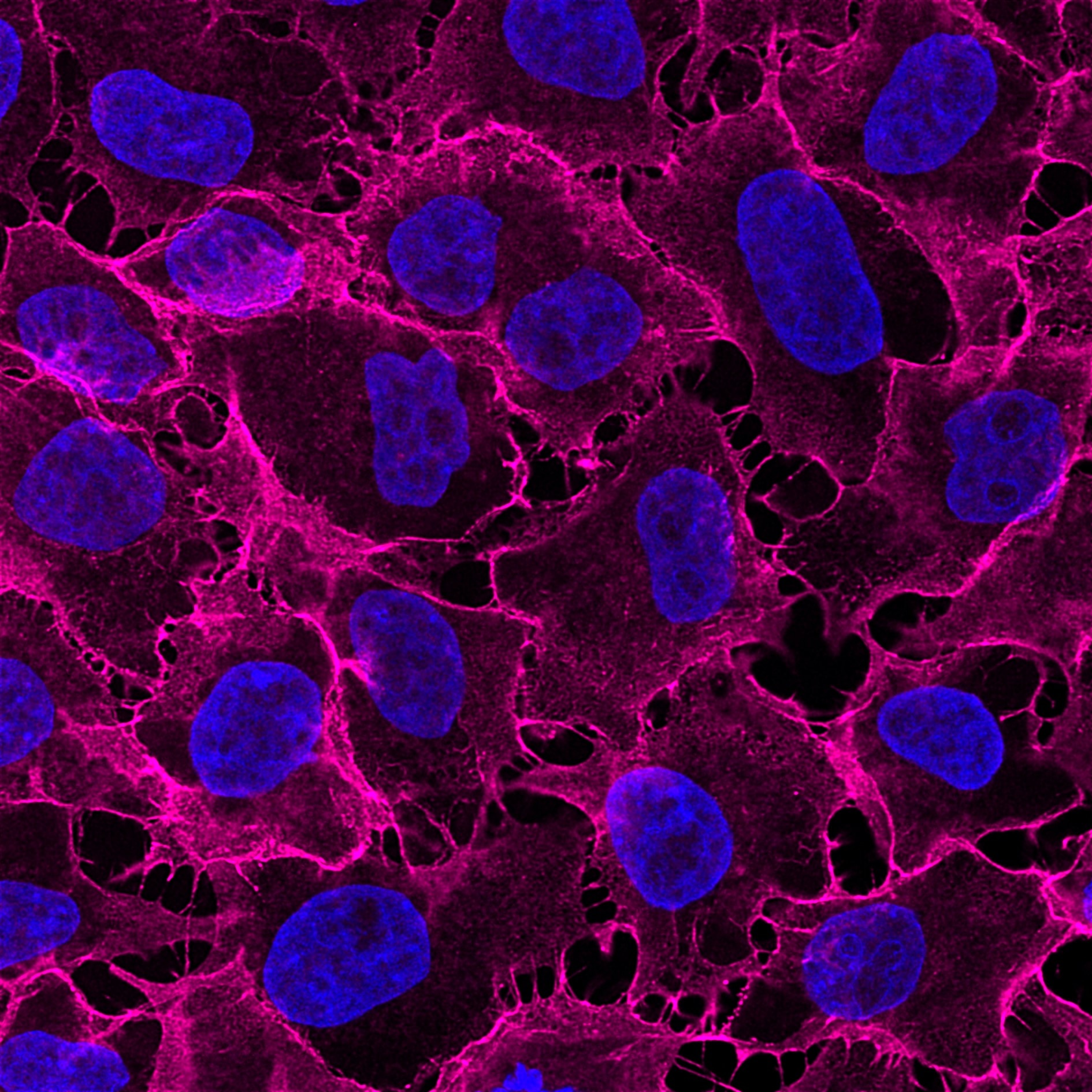
Immunofluorescence analysis of HeLa cells stained with mouse IgG1 anti-CD147 antibody and Nano-Secondary® alpaca anti-mouse IgG1, recombinant VHH, CoraLite® Plus 647 (smsG1CL647-1, magenta). Nuclei were stained with DAPI (blue). Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
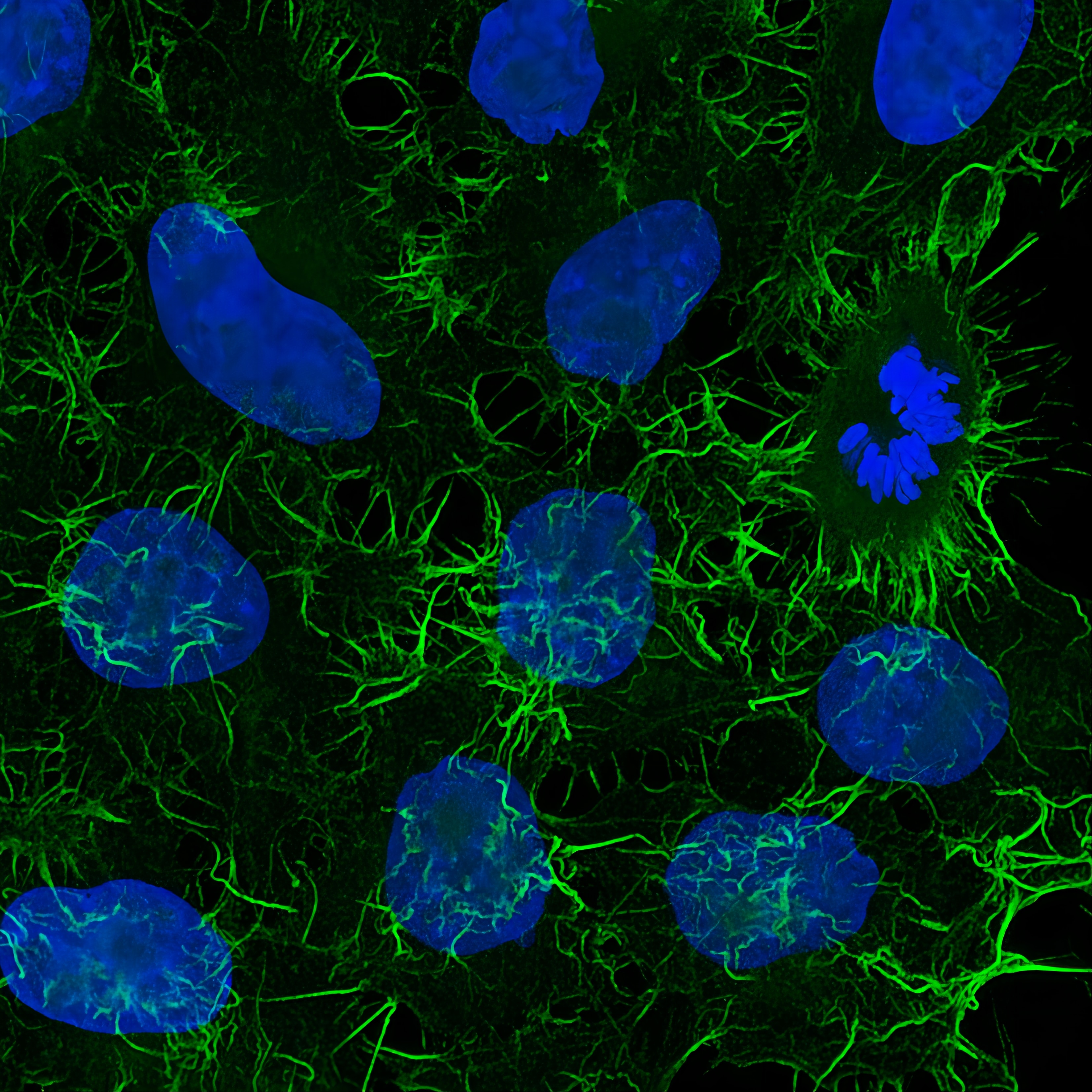
Immunofluorescence analysis of HeLa cells stained with mouse IgG2a anti-Actin antibody and Nano-Secondary® alpaca anti-mouse IgG2a, recombinant VHH, CoraLite® Plus 488 (green) Nuclei were stained with DAPI (blue). Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
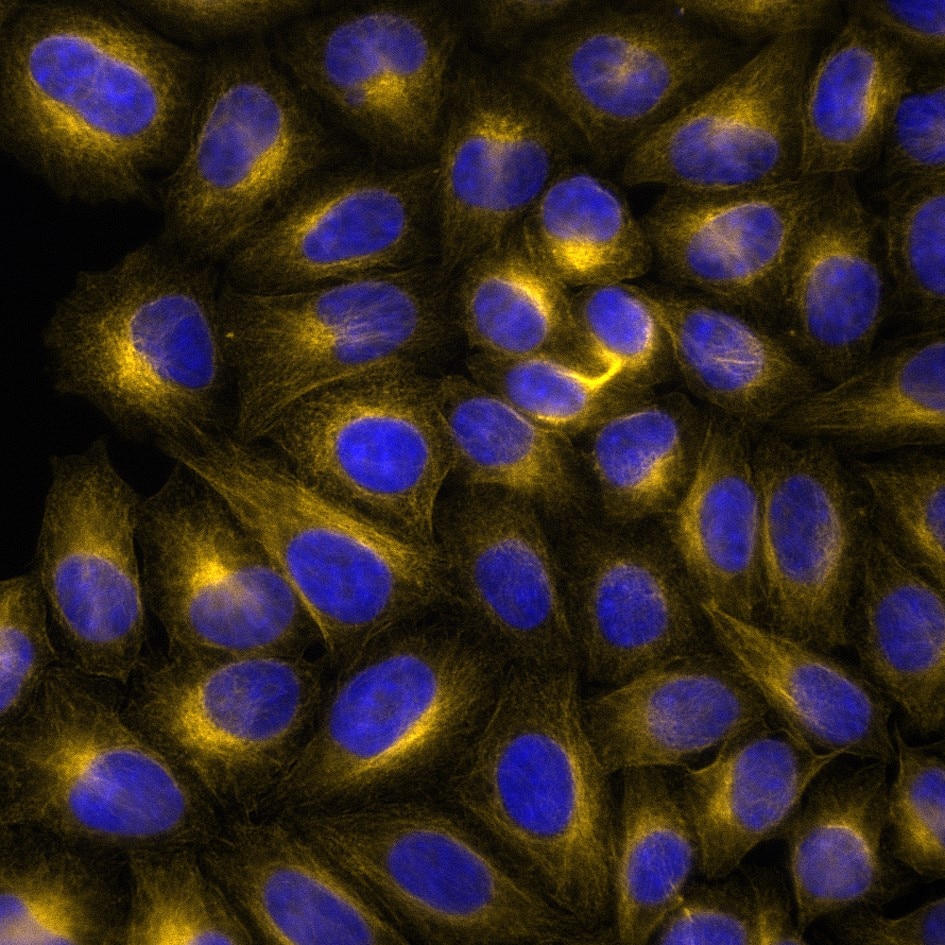
Immunofluorescence analysis of Hela cells stained with mouse IgG2a anti-Tubulin antibody (66240-1-Ig) and Nano-Secondary® alpaca anti-mouse IgG2a, recombinant VHH, CoraLite® Plus 647 (magenta). Nuclei were stained with DAPI (blue). Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
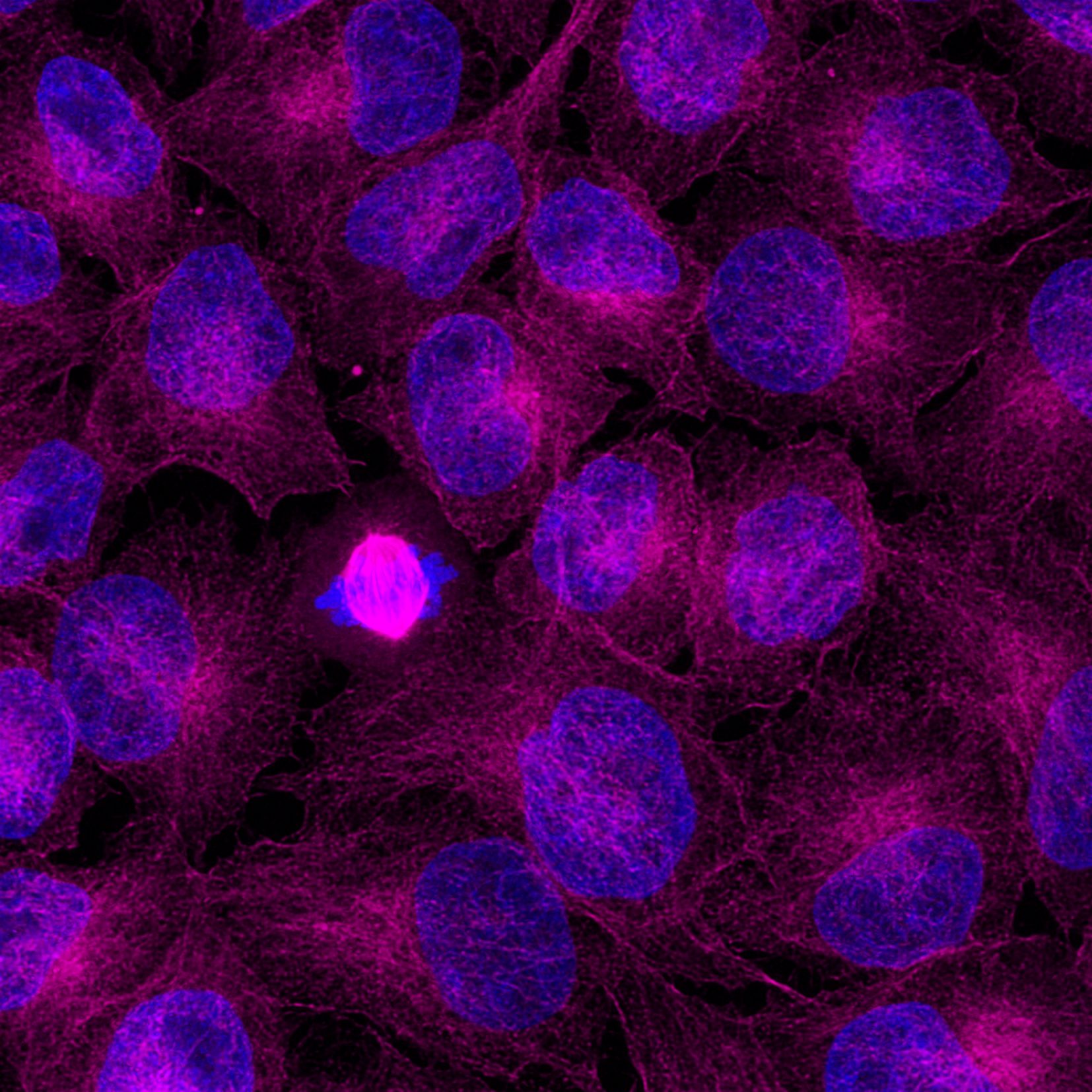
Immunofluorescence analysis of HeLa cells stained with mouse IgG2b anti-Tubulin beta antibody and Nano-Secondary® alpaca anti-mouse IgG2b, recombinant VHH, CoraLite® Plus 555 (smsG2bCL555-1, orange). Nuclei were stained with DAPI (blue). Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
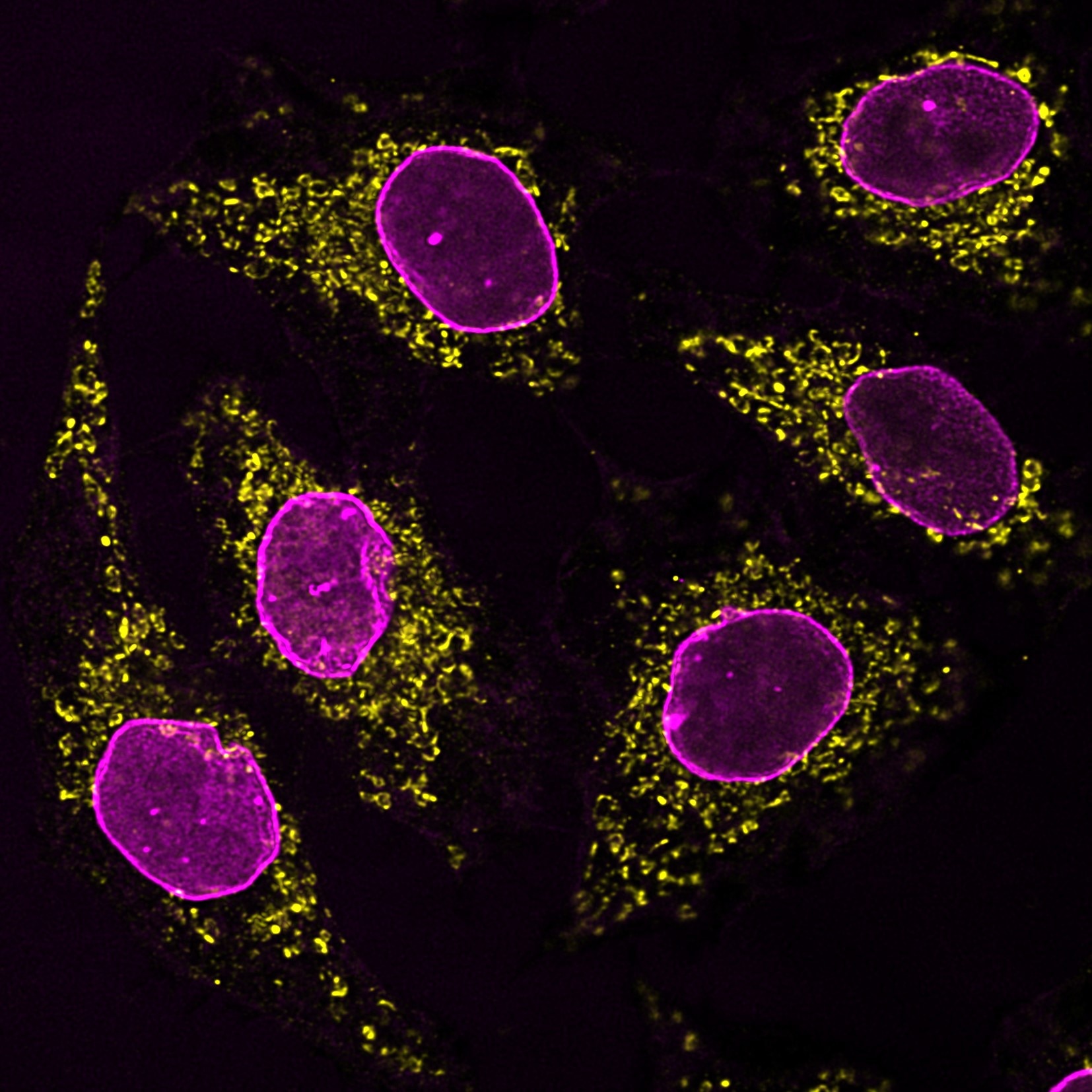
Immunofluorescence analysis of HeLa cells co-stained with mouse IgG1 anti-HSP60 antibody (66041-1-Ig) and mouse IgG2b anti-Lamin A/C antibody followed by Nano-Secondary® alpaca anti-mouse IgG1, recombinant VHH, CoraLite® Plus 555 (smsG1CL555-1, yellow) and Nano-Secondary® alpaca anti-mouse IgG2b, recombinant VHH, CoraLite® Plus 647 (smsG1CL647-1, magenta). Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
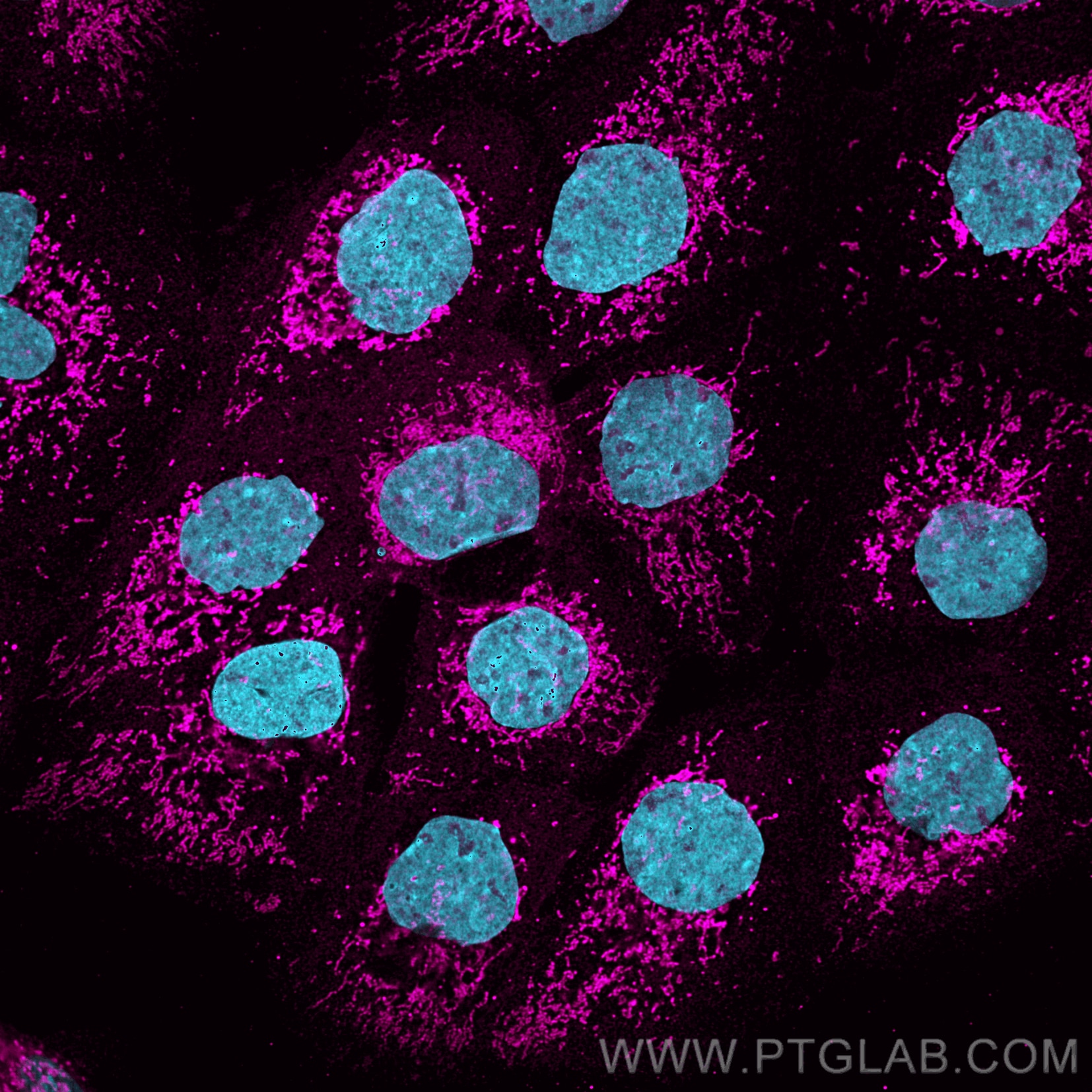
Immunofluorescence analysis of A431 cells stained with rabbit anti-COXIV antibody (11242-1-AP, magenta) and Nano-Secondary® alpaca anti-rabbit IgG, recombinant VHH, CoraLite® Plus 647 (srb2GCL647-1), 1:500. Nuclei were stained with DAPI (blue). Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
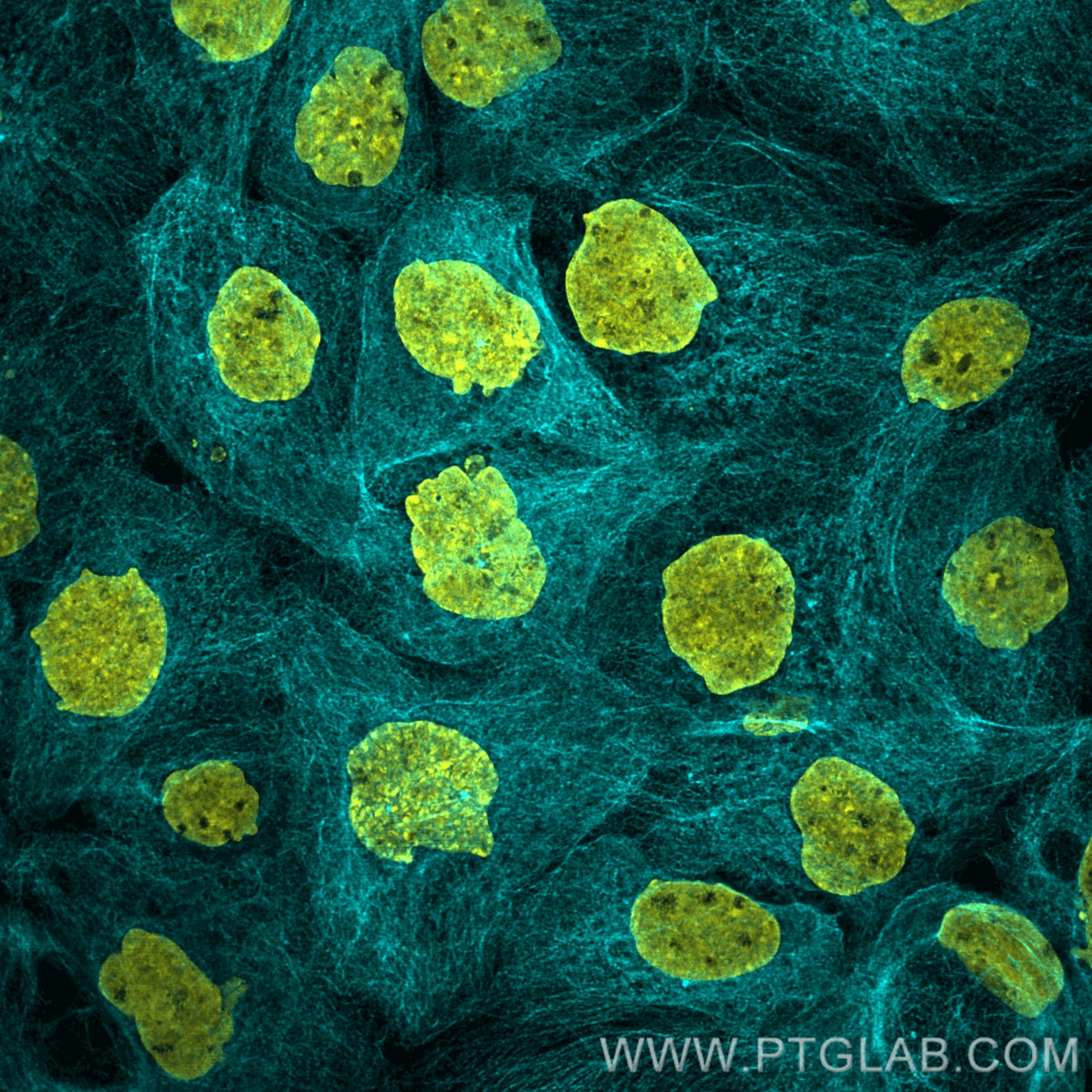
Immunofluorescence analysis of A431 cells stained with rabbit anti-tubulin antibody (80713-1-AP, cyan) and Nano-Secondary® alpaca anti-rabbit IgG, recombinant VHH, CoraLite® Plus 750 (srb2GCL750-1), 1:500. Nuclei were stained with DAPI. Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
![Live A431 cells were incubated with anti-human EGFR (Cetuximab biosimilar) followed by Nano-Secondary® alpaca anti-human IgG, recombinant VHH, CoraLite® Plus 555 [CTK0117] (shuGCL555-2, orange). Cells were fixed and nuclei were stained with DAPI (blue). Epifluorescence images were acquired with a 40x objective and post-processed.](https://www.ptglab.com/products/pictures/Result-shuGCL555-2.jpg)
Live A431 cells were incubated with anti-human EGFR (Cetuximab biosimilar) followed by Nano-Secondary® alpaca anti-human IgG, recombinant VHH, CoraLite® Plus 555 [CTK0117] (shuGCL555-2, orange). Cells were fixed and nuclei were stained with DAPI (blue). Epifluorescence images were acquired with a 40x objective and post-processed.
![Immunofluorescence analysis of transgenic CHO-TIM3 cells. PFA-fixed cells were stained with anti-human TIM3 (Cobolimab biosimilar), Nano-Secondary® alpaca anti-human IgG, recombinant VHH, CoraLite® Plus 647 [CTK0117] (gray) and DAPI (blue).](https://www.ptglab.com/products/pictures/Result-shuGCL647-2_20231123151955368.jpg)
Immunofluorescence analysis of transgenic CHO-TIM3 cells. PFA-fixed cells were stained with anti-human TIM3 (Cobolimab biosimilar), Nano-Secondary® alpaca anti-human IgG, recombinant VHH, CoraLite® Plus 647 [CTK0117] (gray) and DAPI (blue).
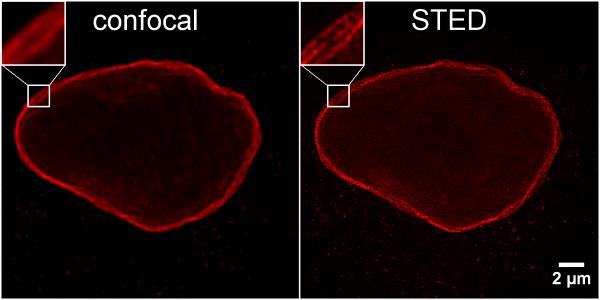

HeLa cells were immunostained with rabbit anti-Lamin B1 antibodies and alpaca anti-rabbit IgG VHH Alexa Fluor® 568 (1:1,000). Confocal and gated STED images were acquired with a Leica TCS SP8 STED 3X microscope, pulsed depletion with a 775 nm laser. Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
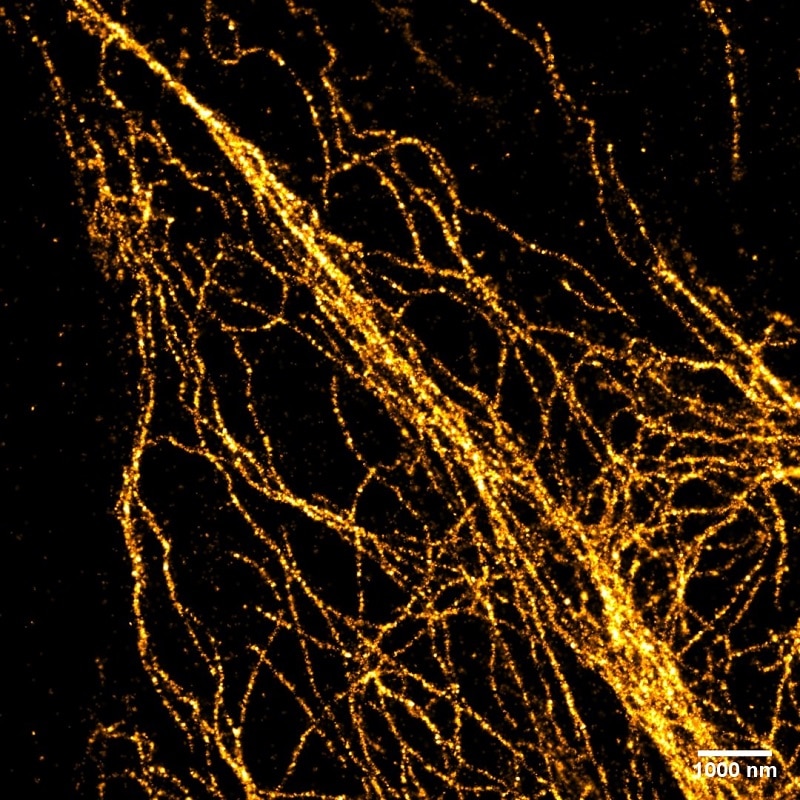
Vimentin in HeLa cells: The cells were stained with monoclonal anti-Vimentin mouse IgG1 antibody and anti-mouse IgG1 VHH Alexa Fluor® 488 and recorded with Leica GSDIM system. The image is a courtesy of Dr. Leila Nahidiazar, Dr. Jop Kind, and Prof. Kees Jalink.
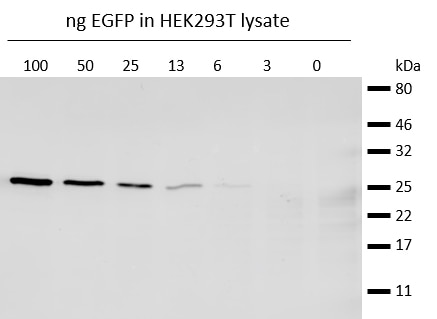

Western blot analysis of EGFP (EGFP-250, ChromoTek) added to HEK293T cell lysate. Detection with anti-GFP mouse IgG 1 antibody and alpaca anti-mouse IgG1 VHH Alexa Fluor® 647.
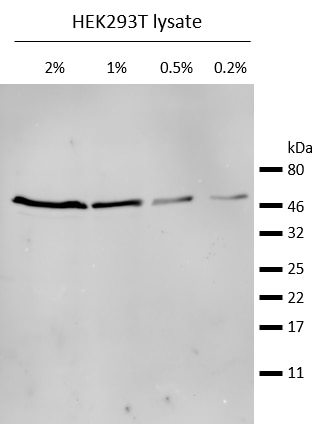
Western blot analysis of endogenous ®-Tubulin in HEK293T cell lysate. Detection with mouse anti-®-Tubulin antibody and alpaca anti-mouse IgG2b VHH Alexa Fluor® 647.
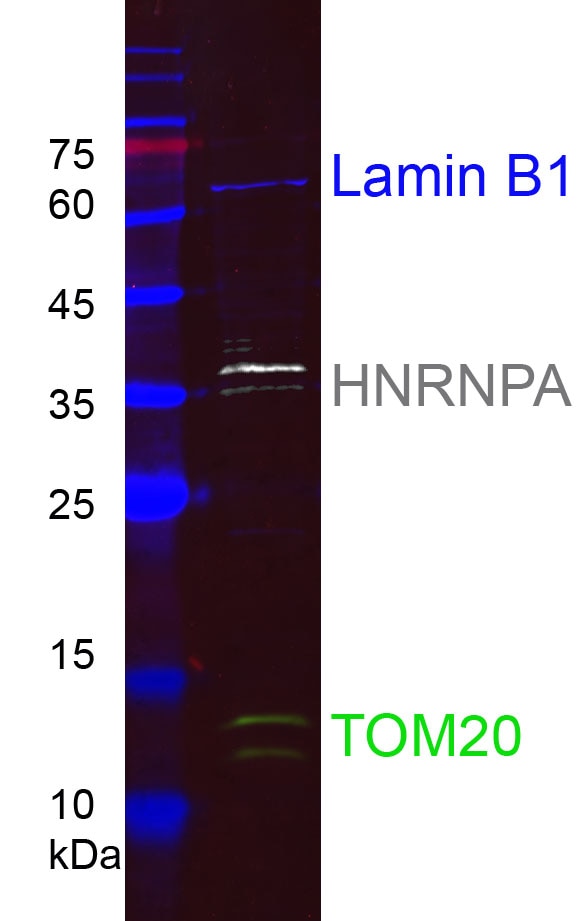
HEK-293 cell lysates were subjected to SDS-PAGE followed by multiplex western blot analysis with 3 mouse primary antibodies including anti-Lamin B1 (66095-1-Ig), anti-HNRNPA (67445-1-Ig), and anti-Tom20 (66777-1-Ig). Primary antibodies were detected using 3 mouse IgG subclass-specific nano-secondary reagents including Nano-Secondary® alpaca anti-mouse IgG1, recombinant VHH, CoraLite® Plus 647 (smsG1CL647-1, blue), Nano-Secondary® alpaca anti-mouse IgG2a, recombinant VHH, CoraLite® Plus 750 (smsG2aCL750-1, white), and Nano-Secondary® alpaca anti-mouse IgG2b, recombinant VHH, CoraLite® Plus 488 (smsG2bCL488-1, green).
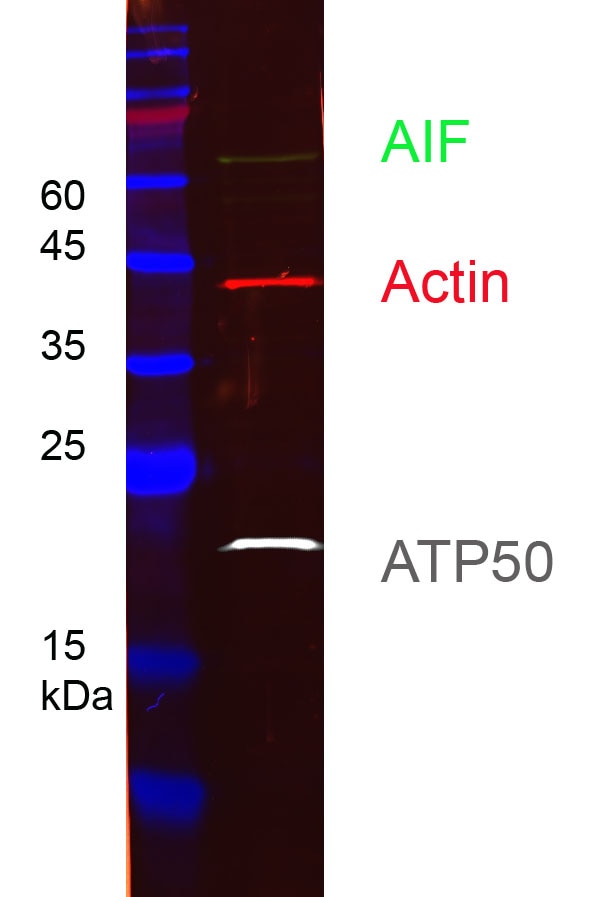
HEK-293 cell lysates were subjected to SDS-PAGE followed by multiplex western blot analysis with 3 mouse primary antibodies including anti-ATP50 (66696-1-Ig), anti-AIF (67791-1-Ig), and anti-actin (66009-1-Ig). Primary antibodies were detected using 3 mouse IgG subclass-specific nano-secondary reagents including Nano-Secondary® alpaca anti-mouse IgG1, recombinant VHH, CoraLite® Plus 750 (smsG1CL750-1, white), Nano-Secondary® alpaca anti-mouse IgG2a, recombinant VHH, CoraLite® Plus 488 (smsG2aCL488-1, green), and Nano-Secondary® alpaca anti-mouse IgG2b, recombinant VHH, CoraLite® Plus 555 (smsG2bCL555-1, red).
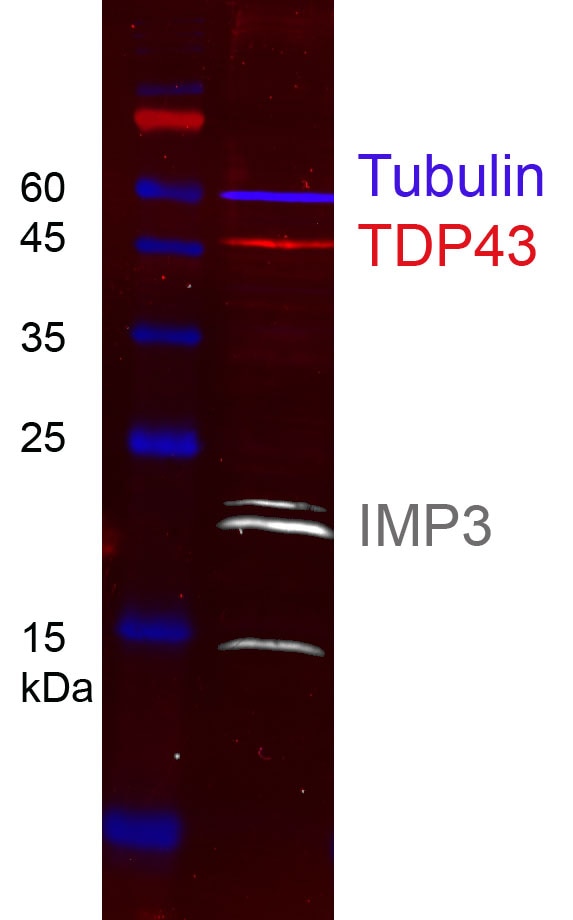
HEK-293 cell lysates were subjected to SDS-PAGE followed by multiplex western blot analysis with 3 mouse primary antibodies including anti-TDP43 (60019-1-Ig), anti-tubulin (66240-1-Ig), and anti-IMP3 (66247-1-Ig). Primary antibodies were detected using 3 mouse IgG subclass-specific nano-secondary reagents including Nano-Secondary® alpaca anti-mouse IgG1, recombinant VHH, CoraLite® Plus 555 (smsG1CL555-1, red), Nano-Secondary® alpaca anti-mouse IgG2a, recombinant VHH, CoraLite® Plus 647 (smsG2aCL647-1, blue), and Nano-Secondary® alpaca anti-mouse IgG2b, recombinant VHH, CoraLite® Plus 750 (smsG2bCL750-1, white).
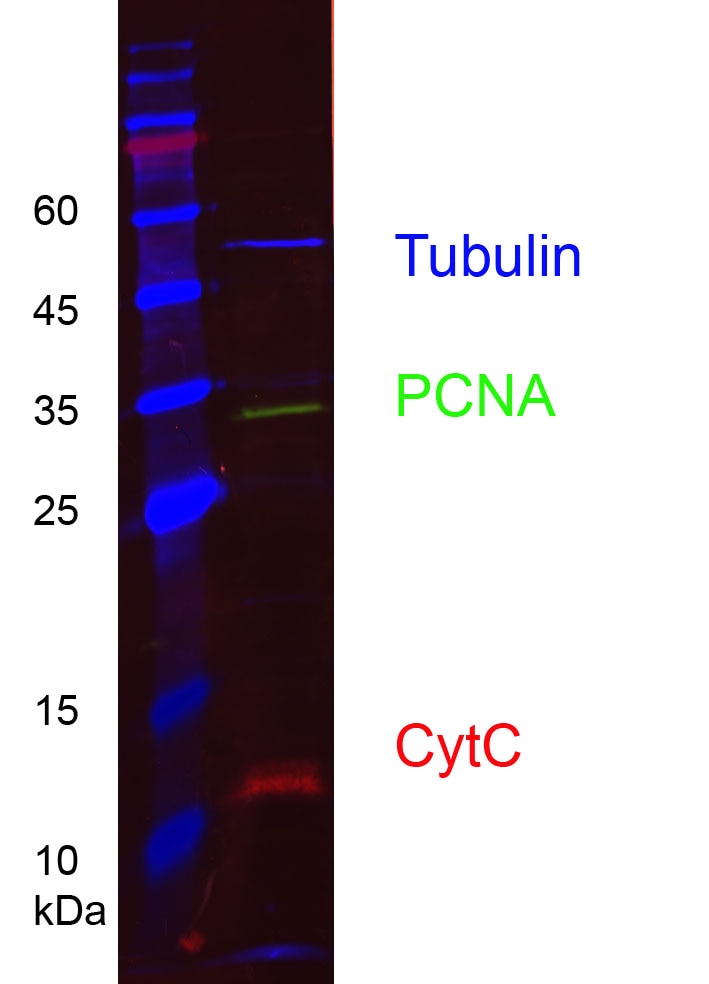
HEK-293 cell lysates were subjected to SDS-PAGE followed by multiplex western blot analysis with 3 mouse primary antibodies including anti-PCNA (60097-1-Ig), anti-cytochrome C (66264-1-Ig), and anti-alpha tubulin (66031-1-Ig). Primary antibodies were detected using 3 mouse IgG subclass-specific nano-secondary reagents including Nano-Secondary® alpaca anti-mouse IgG1, recombinant VHH, CoraLite® Plus 488 (smsG1CL488-1, green), Nano-Secondary® alpaca anti-mouse IgG2a, recombinant VHH, CoraLite® Plus 555 (smsG2aCL555-1, red), and Nano-Secondary® alpaca anti-mouse IgG2b, recombinant VHH, CoraLite® Plus 647 (smsG2bCL647-1, blue).
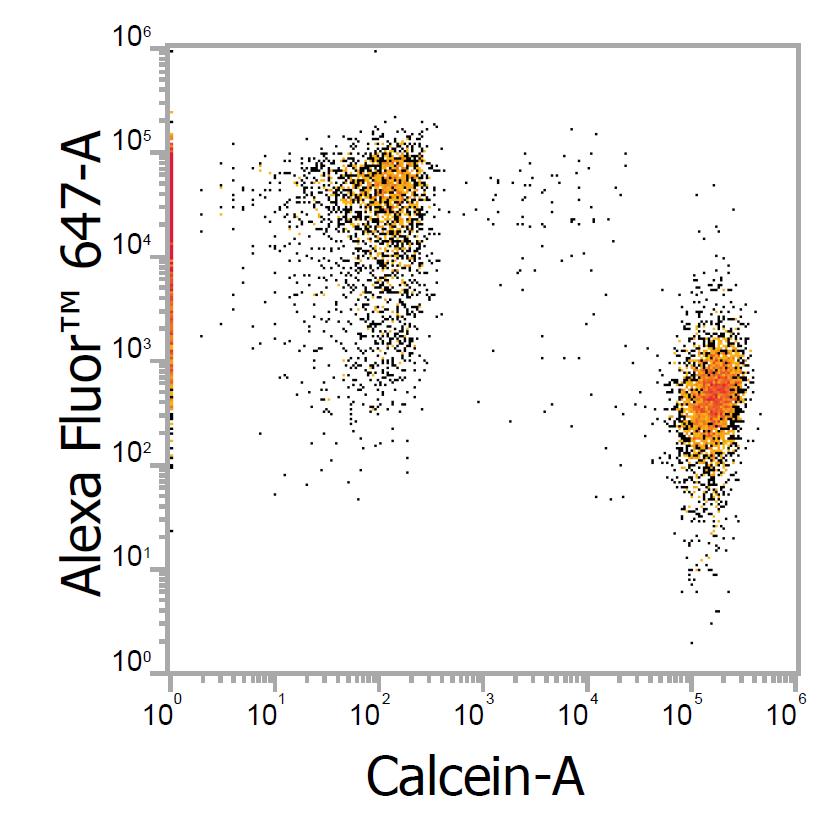
Immunostaining with Alpaca anti-mouse IgG1 Alexa Fluor 647 Nano-Secondaries for FACS. Jurkat CD3+ and Jurkat CD3- cell lines were mixed and immunostained live with anti-CD3 mouse IgG1 + alpaca anti-mouse IgG1 VHH Alexa Fluor 647 (1:600). CD3- cells were pre-stained with Calcein. Two cell populations can be clearly distinguished on the dot-plot: Alexa Fluor 647-positive Calcein-negative cells and Alexa Fluor 647-negative Calcein-positive cells.
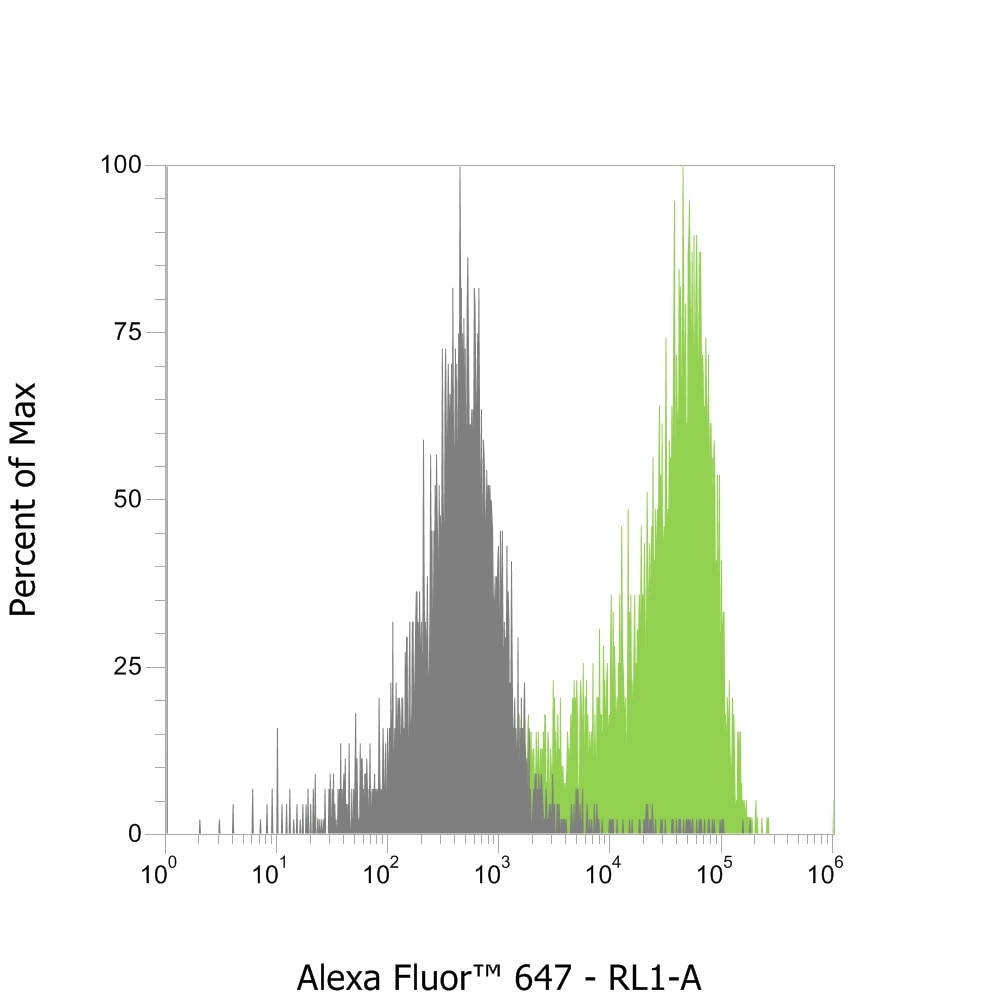
Flow cytometry with anti-mouse Nano-Secondaries. Jurkat CD3+ and Jurkat CD3- cell lines were mixed and immunostained live with anti-CD3 mouse IgG1 and alpaca anti-mouse IgG1 VHH Alexa Fluor® 647 (1:600). Two cell populations can be clearly distinguished with a 2-log shift: CD3-positive cells (in green) and CD3-negative cells (in grey).
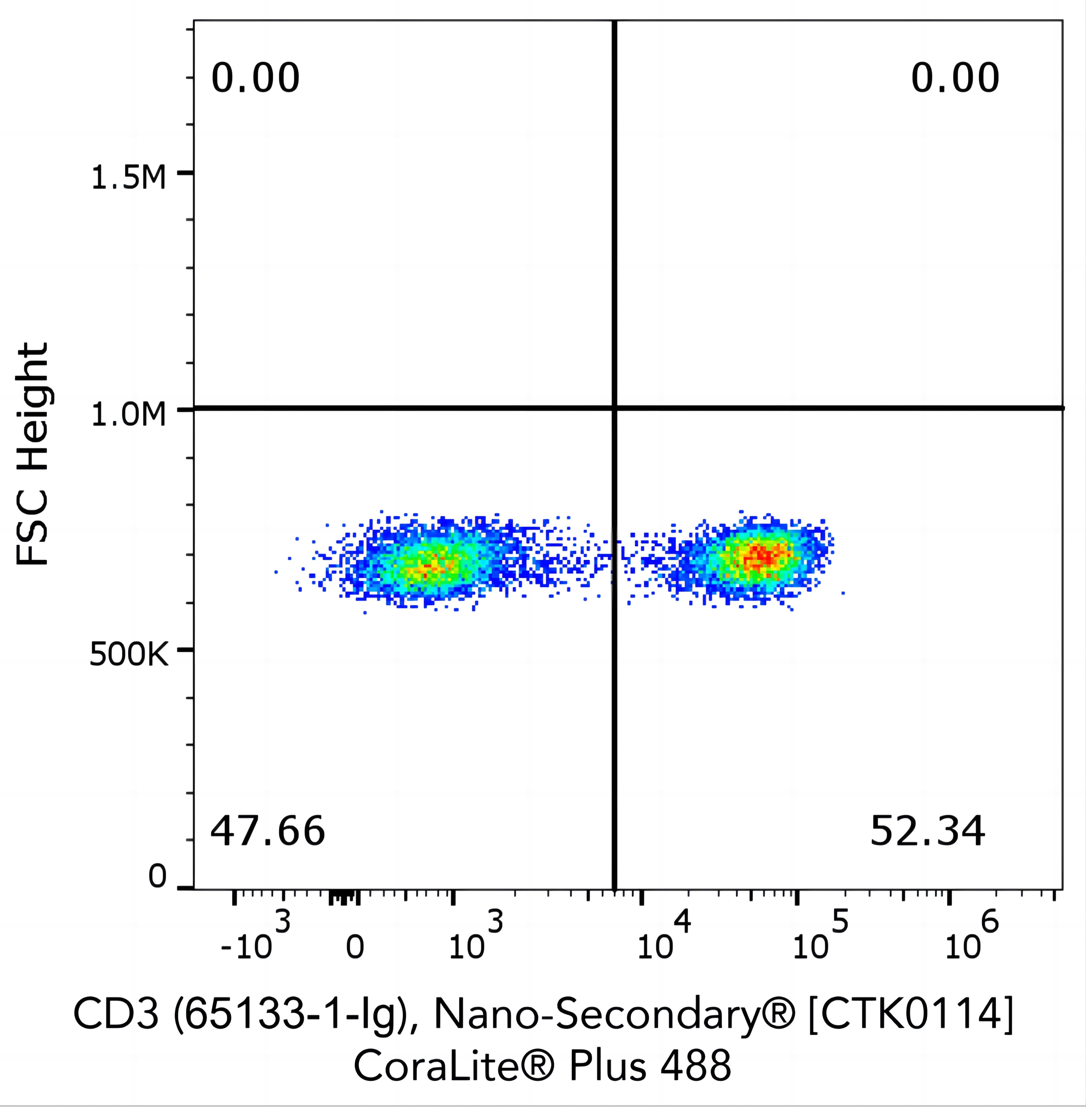
Flow cytometry analysis of 1X10^6 human peripheral blood mononuclear cells (PBMCs) stained with anti-human CD3 (clone OKT3, 65133-1-Ig) and Nano-Secondary® alpaca anti-mouse IgG2a, recombinant VHH, CoraLite® Plus 488.
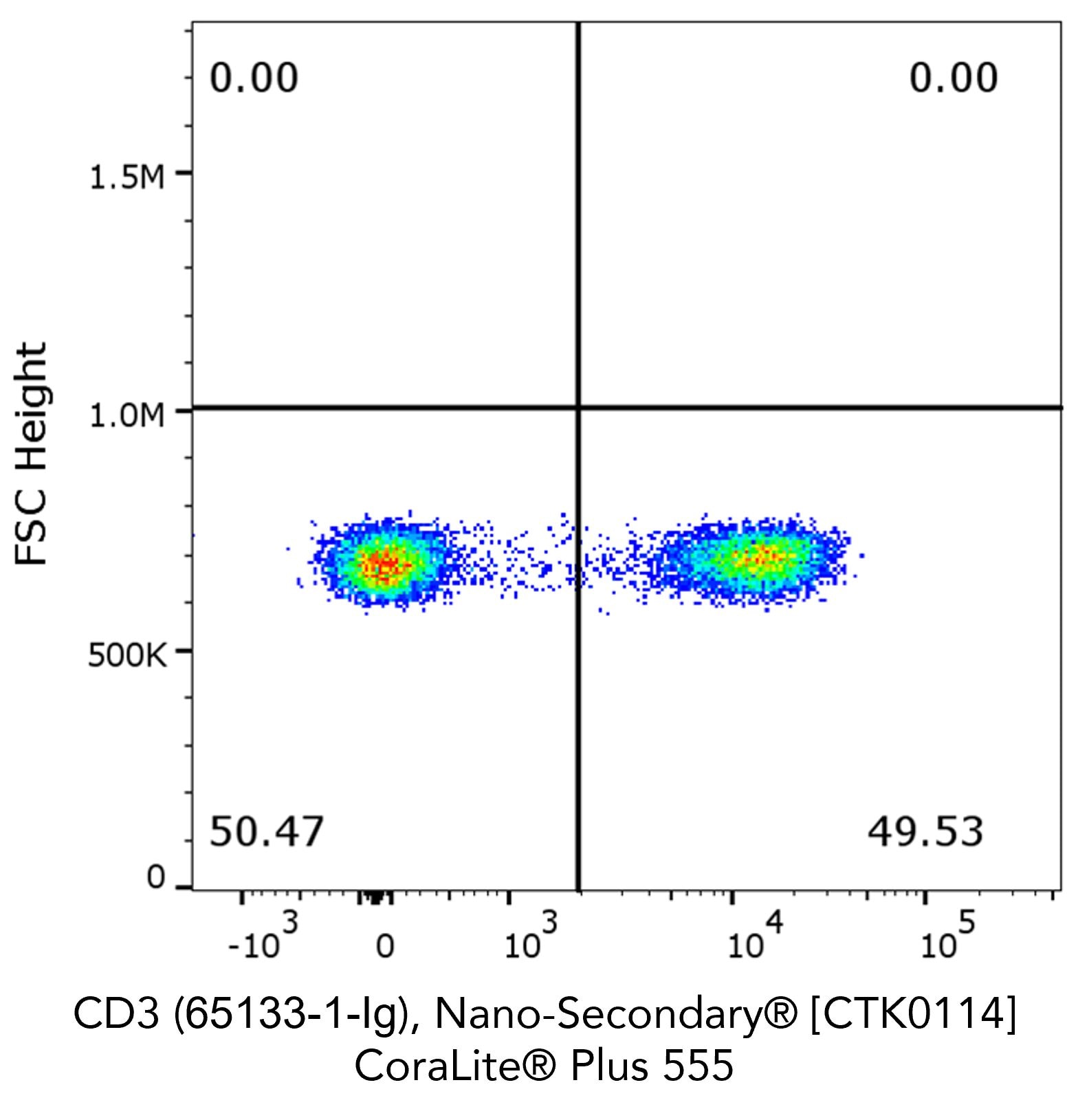
Flow cytometry analysis of 1X10^6 human peripheral blood mononuclear cells (PBMCs) stained with anti-human CD3 (clone OKT3, 65133-1-Ig) and Nano-Secondary® alpaca anti-mouse IgG2a, recombinant VHH, CoraLite® Plus 555.
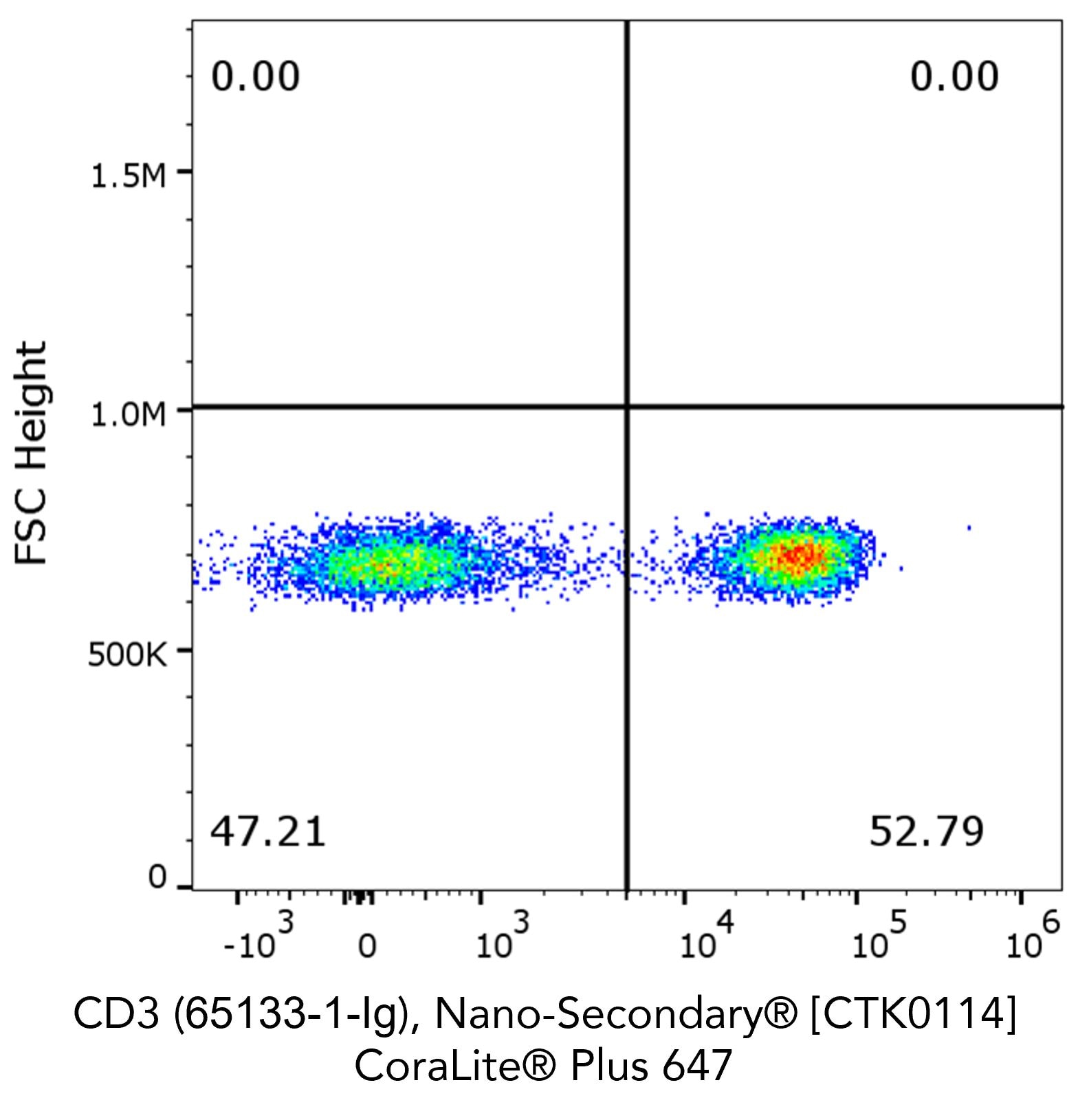
Flow cytometry analysis of 1X10^6 human peripheral blood mononuclear cells (PBMCs) stained with anti-human CD3 (clone OKT3, 65133-1-Ig) and Nano-Secondary® alpaca anti-mouse IgG2a, recombinant VHH, CoraLite® Plus 647.
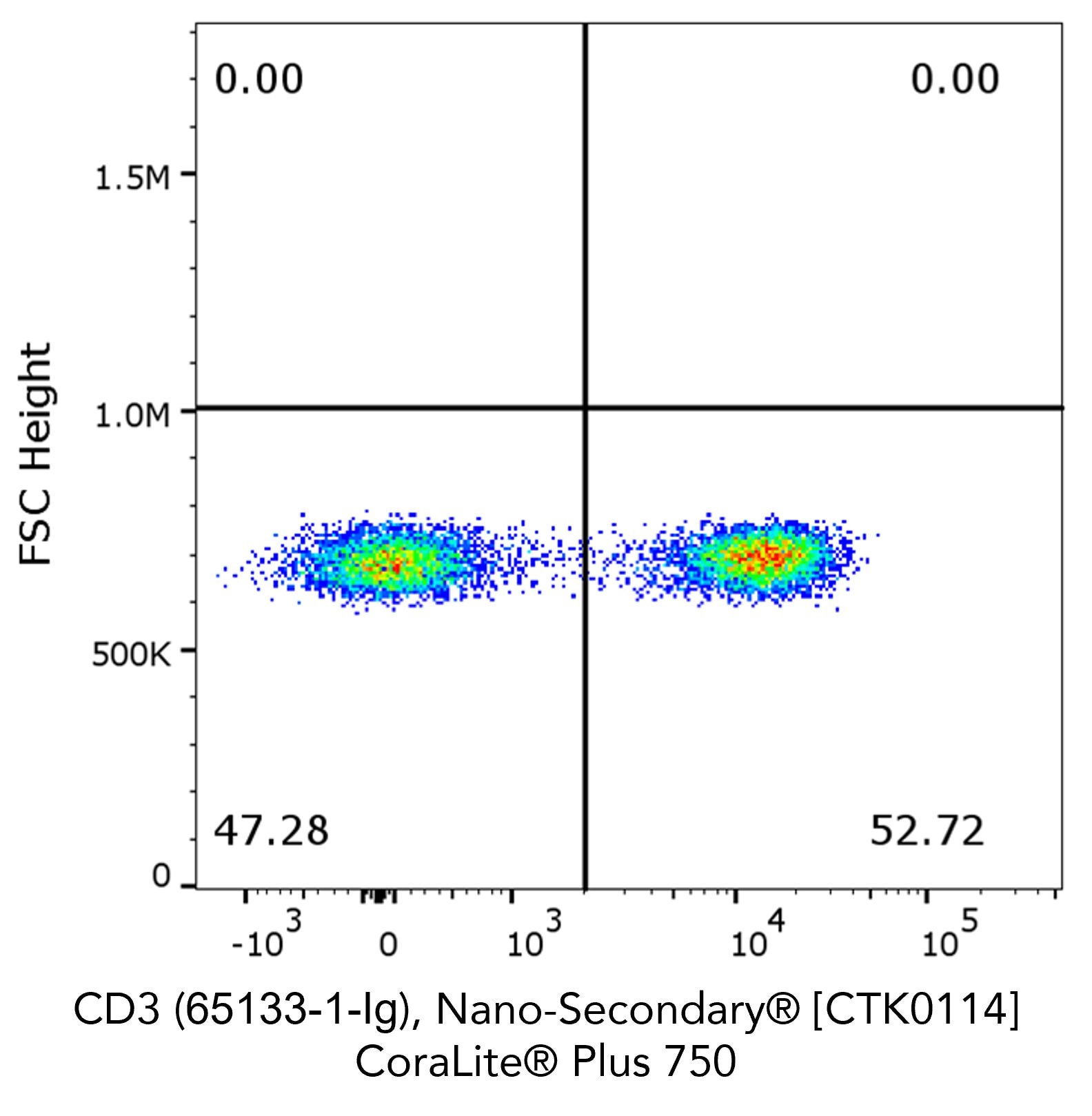
Flow cytometry analysis of 1X10^6 human peripheral blood mononuclear cells (PBMCs) stained with anti-human CD3 (clone OKT3, 65133-1-Ig) and Nano-Secondary® alpaca anti-mouse IgG2a, recombinant VHH, CoraLite® Plus 750.
Our Nano-Secondary reagents are about 15 kDa in size and thus 10 times smaller than conventional secondary antibodies or IgGs, which are ≥ 150 kDa. That small size considerably improves tissue penetration and minimizes the distance between epitope and label, which increases imaging resolution and can make signals less diffuse. Hence, Nano-Secondary reagents are perfectly suited for super-resolution microscopy techniques like STED, and STORM.

HeLa cells were immunostained with rabbit anti-Lamin B1 antibodies and alpaca anti-rabbit IgG VHH Alexa Fluor® 568 (1:1,000). Confocal and gated STED images were acquired with a Leica TCS SP8 STED 3X microscope, pulsed depletion with a 775 nm laser. Images were recorded at the Core Facility Bioimaging at the Biomedical Center, LMU Munich.
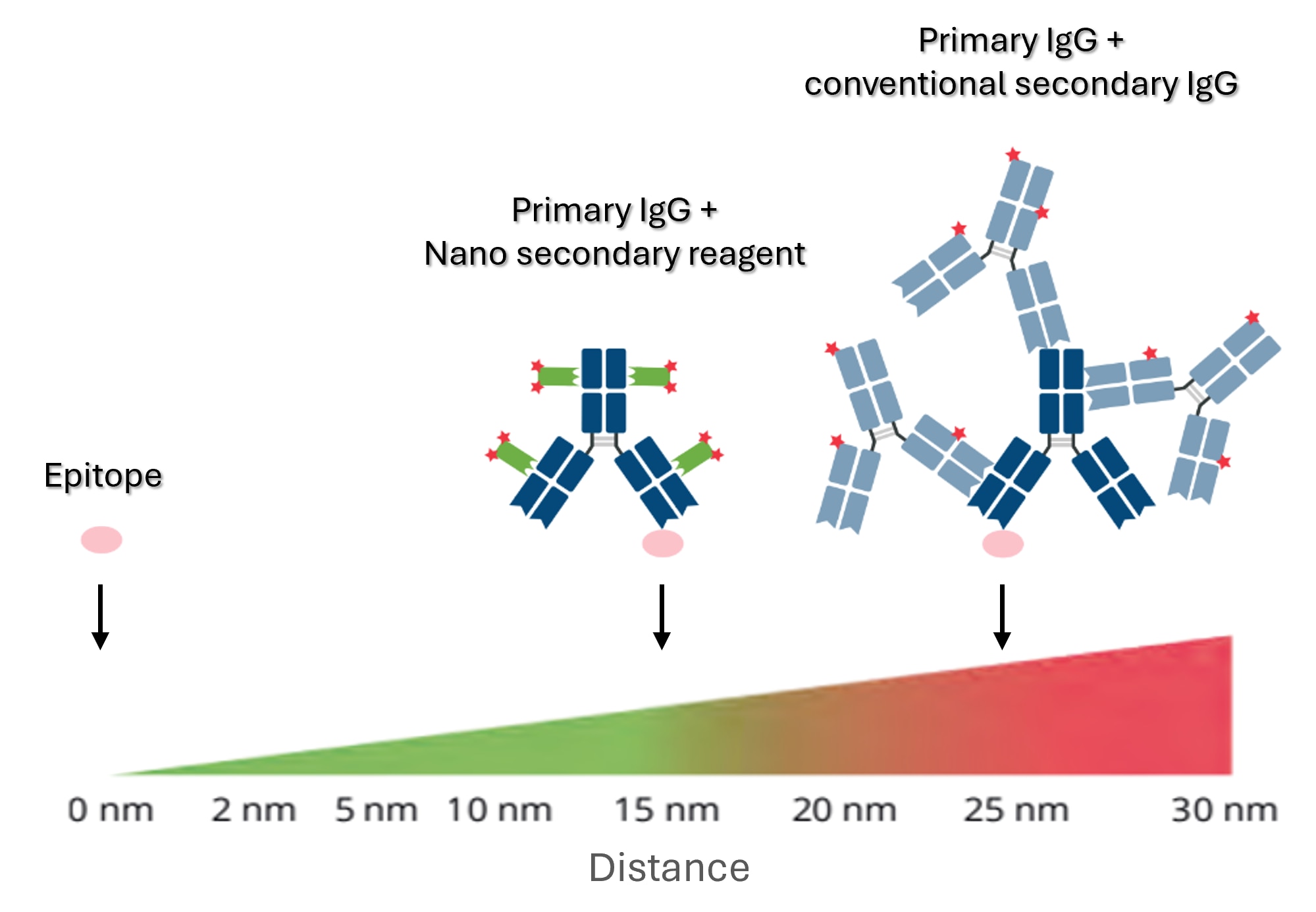
Left: primary IgG (dark blue) & Fab and Fc specific Nano secondary reagents (green) with two labels each (red). Right: primary antibody + polyclonal secondary IgG (light blue). The epitope is shown in pink. Epitope label displacement is reduced to a minimum with Nano- Secondary reagents.
During product development we select only Nanobodies with the desired specificity. Nanobody clones with cross-reactivity to other, commonly used species’ IgG or serum proteins are excluded. Therefore, our Nano-Secondary reagents have a very low background and do not require any kind of pre-adsorption. Currently, we offer Nano-Secondary reagents against human IgG, rabbit IgG, and mouse IgG subclasses 1, 2a, 2b, and 3, enabling multiplexing of multiple mouse primary antibodies.
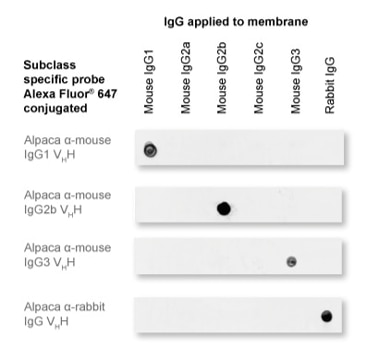
Our nano secondary reagents are subclass-specific and do not cross-react with IgGs from other commonly used species or even same species IgG subtypes.
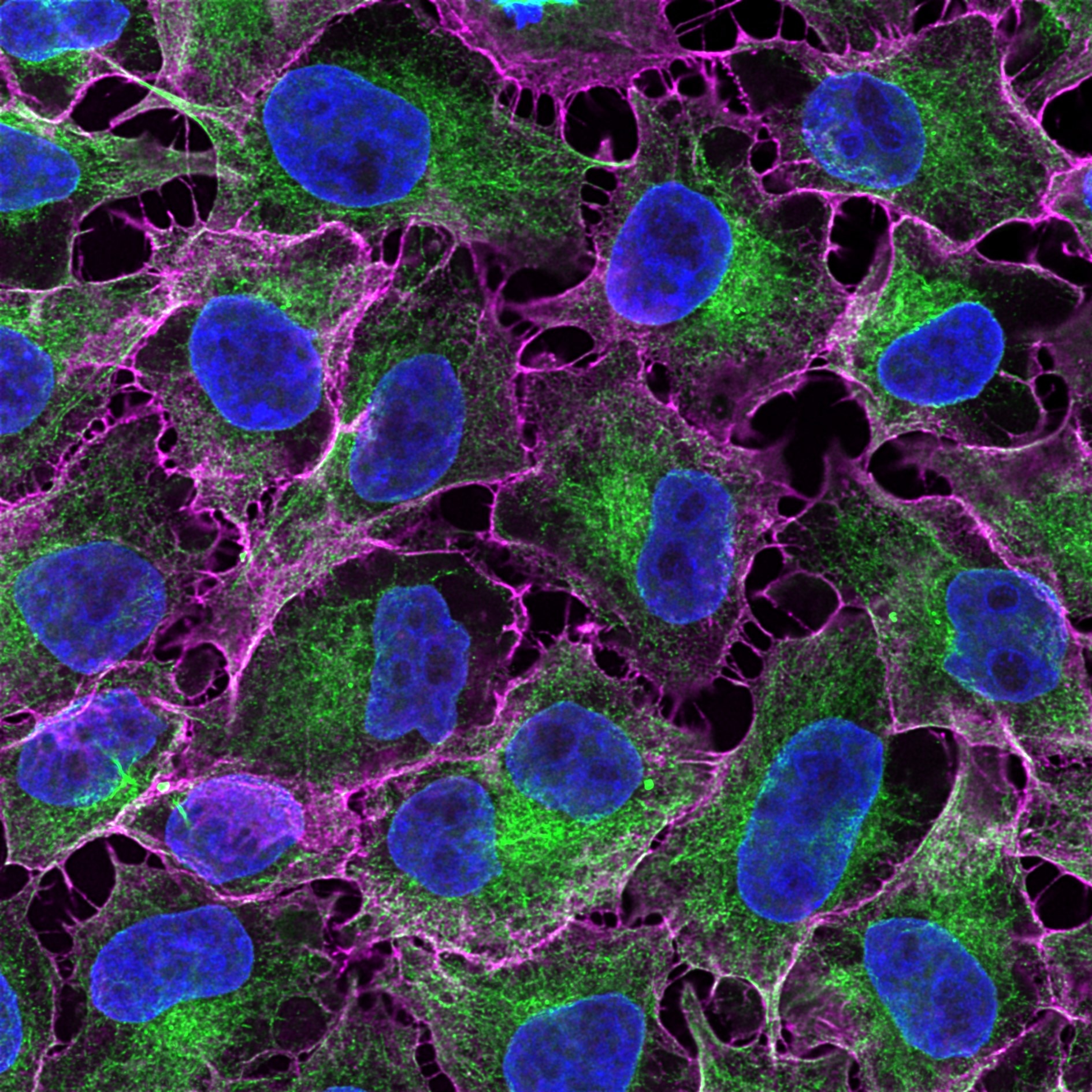
Immunofluorescence analysis of HeLa cells co-stained with mouse IgG2b anti-tubulin beta antibody and mouse IgG1 CD147 antibody followed by Nano-Secondary® alpaca anti-mouse IgG2b, recombinant VHH, CoraLite® Plus 488 (smsG2bCL488-1, green) and Nano-Secondary® alpaca anti-mouse IgG1, recombinant VHH, CoraLite® Plus 647 (smsG1CL647-1, magenta). Nuclei were stained with DAPI (blue).
ChromoTek’s recombinant Nano-Secondary reagents are characterized by both DNA and protein sequence. Therefore, we generate identical Nano-Secondary reagents in each manufacturing batch with great purity, and virtually no batch-to-batch variation. The production process of our nanobodies is completely animal free. Through site specific conjugation, we can produce nanobodies with a defined degree of labeling. We validate each batch i.a. with UV/VIS spectroscopy, SDS-PAGE, IF, and mass-spectrometry.
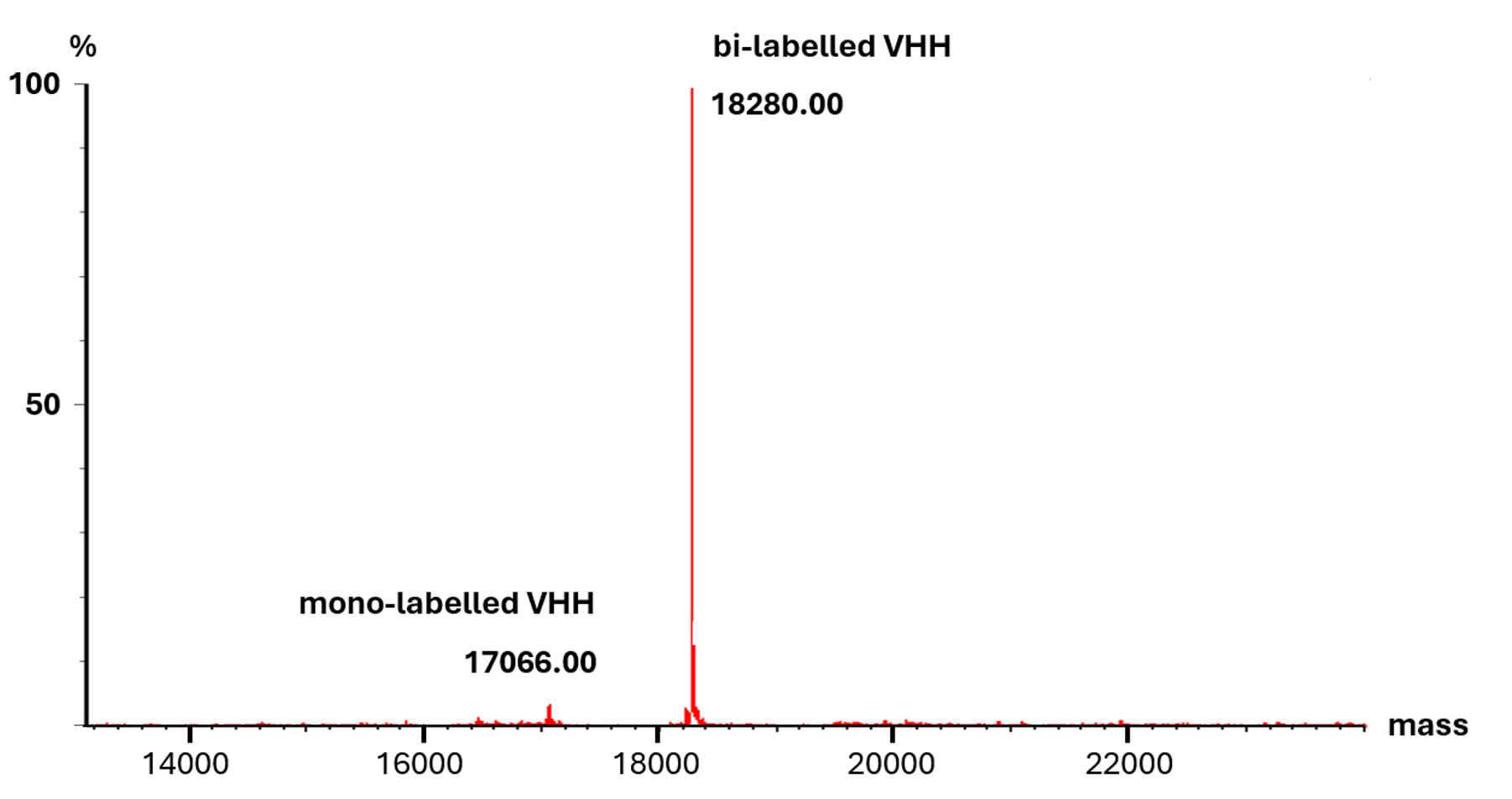
ESI-TOF intact weight mass spectrometry validation of the degree of labeling of a Nano-Secondary after site-directed conjugation. The main peak corresponds to the Nano-Secondary with two dyes (expected molecular weight of 18278 Da), indicating >95 % complete and successful labeling.

