TransAM® Flexi NFκB Kits
TransAM-Flexi-NF-B-Kits-AM220
Synonyms
| Name | Format | Cat No. | Price | Delivery |
|---|
Overview
TransAM Flexi Kit Overview
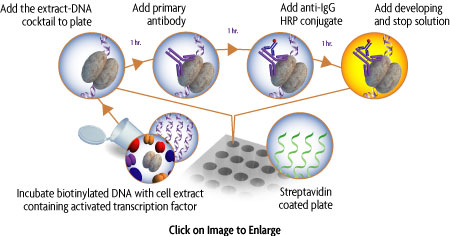
TransAM Flexi NFκB Kits provide everything needed to study Nuclear Factor κB (NFkB), including a positive control extract and positive control biotinylated oligonucelotide. The TransAM Flexi format enables you to look at NFκB binding sites of your own design. This kit works by combining a stimulated nuclear extract with biotinylated oligos you generated followed by transfer to a 96-well streptavidin coated plate to capture activated NFκB binding. Kits are available with antibodies specific for the activated form of p50 and p65. A family kit is also available with the ability to screen the members of the NFkB family (p50, p52, p65, c-Rel and RelB). Recombinant p50 and Recombinant p65 proteins are available separately to generate an optional protein standard curve in the TransAM NFκB Transcription Factor ELISA kits.

Measuring NFκB p65 at different binding sites.Five µg of nuclear extract from untreated and TNF-α-treated HeLa cells were used to assay the binding affinity of NFκB p65 for four different biotinylated 50-mer oligos. Each of the 3 test oligos (Igκ, IL-8 and uPA) contained the wild-type binding site of a promoter regulated by NFκB. These sites were compared to the consensus-binding site control provided in TransAM Flexi NFκB Kits.
The TransAM® Flexi Transcription Factor ELISA Advantage
Historically, transcription factor studies have been conducted using gelshift, Western blot and reporter plasmid transfections, which are time-consuming, do not allow for high-throughput and provide only semi-quantitative results. TransAM assays are up to 100 times more sensitive than gelshift techniques, and can be completed in less than 5 hours. Because TransAM is an ELISA-based assay*, there is no radioactivity, and the high-throughput stripwell format enables simultaneous screening of 1-96 samples. Inconsistencies due to variable reporter plasmid transfections are eliminated, along with the need to construct stable cell lines.
Why use TransAM® Flexi transcription factor ELISAs?
- Up to 100-fold more sensitive than gelshift assays
- Eliminates the use of radioactivity and the need to run gels
- Results in less than five hours
- Colorimetric readout enables easy, quantitative analysis with spectrophotometry at 450nm
- 96-stripwell format enables both high and low throughput
TransAM Flexi Kit applications include:
- Study of variant transcription factor binding sites
- Analysis of native promoters
- Confirmation of chromatin immunoprecipitation (ChIP) results
- Determination of isoform-binding affinity for NFκB
TransAM® Flexi Transcription Factor ELISA Kits
TransAM Flexi Kits are perfect for researchers interested in studying alternative binding sites for their transcription factor. In TransAM Flexi, the researcher synthesizes a biotinylated oligo or PCR product, which contains the transcription factor-binding site of choice. A stimulated nuclear extract is mixed with this biotinylated oligo and then transferred to the wells of a 96-well streptavidin coated plate, where the biotinylated oligo containing the activated transcription factor is captured. Next, a primary antibody specific for an epitope on the bound and active form of the transcription factor is added followed by subsequent incubation with secondary antibody and Developing Solution to provide an easily quantified, sensitive colorimetric readout (Figure 1).
Contents
TransAM Flexi Kit Contents
TransAM Flexi Kits are designed for customers interested in studying alternative binding sites of NFκB and require you to design and synthesize your own oligos of interest, which will then be coated onto the plate. Enough control biotinylated oligo, which contains the consensus-binding site used in the original TransAM NFκB Kits, is provided to perform 40 control reactions. If you are interested in looking at only the consensus site of NFκB, please use the original TransAM NFκB Kits, as they conveniently provide the oligo precoated to the plate. Flexi Kits also include one or two 96-well streptavidin-coated assay plate(s) with plate sealer(s), NFκB p50 or p65 or p50, p52, p65, c-Rel and RelB primary antibody(ies), HRP-conjugated secondary antibody, NFκB wild-type and mutated oligonucleotides, positive control cell extract, DTT, Protease Inhibitor Cocktail, Herring Sperm DNA, Binding, 10X Washing and 10X Antibody Binding Buffers, and Developing Solutions. Reagent storage conditions vary from room temperature to -80°C, see manual for details. All reagents are guaranteed stable for 6 months when stored properly.
NOTE: the p50 and p52 antibodies can be used with human and mouse extracts, while the p65 and RelB antibodies can be used with human, mouse and rat extracts. The c-Rel antibody can only be used with human extracts.
FAQs
TransAM Flexi Kit FAQs
Can I use your TransAM kit with my species of sample?
All of the TransAM kits will work with human samples. Some have cross-reactivity with mouse or rat. Each TransAM manual contains cross-reactivity information under the Kit Performance and Benefits section.
What is the recommended developing time for the colorimetric TransAM assays?
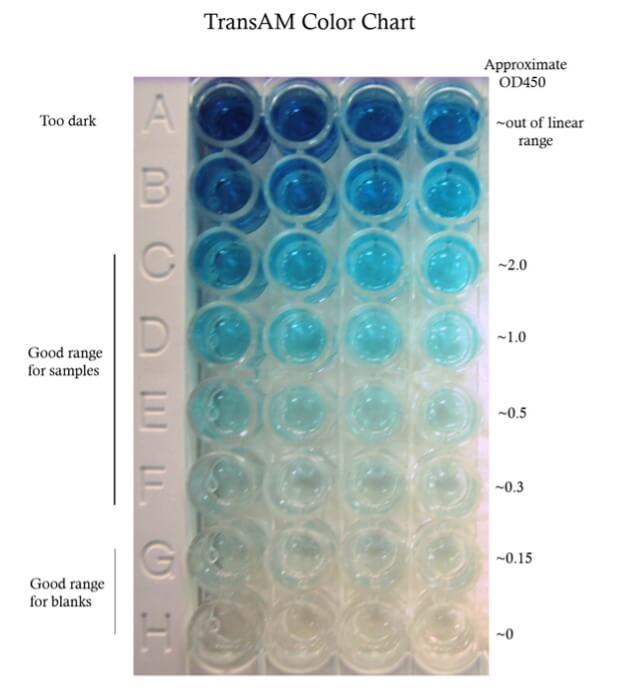
A recommended development time is given in the technical data sheet (TDS) for each lot. However, this time is only a guideline. It is important to monitor the color development for your particular samples. The TransAM color chart gives some guidelines for colorimetric assays.
What is the minimum amount of extract (and minimum number of cells) I can use per well?
Each of our specific TransAM kits has a different detection limit, which is indicated in each manual. For example, our TransAM NF-kB kits can detect binding from as little as 0.5 ug nuclear extract. Using a rough estimate yield of 20 ug nuclear extract from 1 million cells from our Nuclear Extract Kit, 0.5 ug nuclear extract can be obtained from approximately 25,000 cells.
How do I determine how much lysate to use per well?
The optimal protein concentration must be empirically determined. We recommend performing a small titration experiment– 5 ug, 10 ug, 20 ug for nuclear extract, more for whole cell extract, in order to ensure that the amount used per well is within detectable levels. You want to get both the treated and control sample signals within the linear range of the assay. See the TransAM color chart.
Can I increase the volume of sample added per well for the TransAM assay?
Yes, you can increase the total volume of sample added and scale up the reaction, but the ratio of Complete Binding Buffer to Complete Lysis Buffer must remain the same. Please note that there may not be enough reagents to perform all 96-rxns if you scale up and do not exceed a total volume of 100 µl. (Complete Binding Buffer plus sample diluted in Complete Lysis Buffer)
Why do I need to read the absorbance at both OD450nm and OD655nm?
The purpose of the reference wavelength (OD655nm) is to correct or normalize any differences in absorbance that are not due to the analyte. Everything being equal, the reference absorbance should not change between samples. But because of factors like variability in detection equipment, wells, etc. the absorbance can vary. Subtracting the reference from the specific reading: OD450nm - OD655nm accounts for these variations. Often the spectrophotometer or the program automatically does this.
Why do I need to run this assay in duplicates?
We recommend at a minimum running the assay in duplicate to get an idea of technical variability. If your assay has a high variability, we recommend doing triplicates. Plot the data with standard error to get a measure of the variability.
Is a standard curve required?
A standard curve may be used to determine the concentration of the target in your samples. However, determining the exact concentration is not required. In fact, a relative comparison between treated and control samples can be more informative than just the absolute concentration. Another reason to utilize a standard curve is to determine the correlation between protein concentration and the detected signal and ensure that samples fall within the linear range. With the TransAM kits, this range has already been determined and as long as your signals read below 2.0 OD450, the samples are within the linear range of the microplate reader’s detectability.
How do I know if the standard curve I generated is reasonable?
The R² value of the linear regression line should be 0.94 or higher for the standard curve. To increase this value, perform more replicates and pipet with precision.
What is the difference between the colorimetric and the chemiluminescent versions of the TransAM kits?
Generally speaking, a chemiluminescent ELISA is more sensitive and offers a greater dynamic range as compared to colorimetric ELISAs.
What’s the difference between the TransAM family kits and the TransAM individual kits?
Our TransAM family kits come with two 96-well plates and antibodies against multiple transcription factors within a family. Each antibody is provided in enough abundance to cover one whole 96-well plate, so there is flexibility to assay two or more related antibodies. Our TransAM individual kits each come with one 96-well plate and enough of one antibody to cover that plate.
What is the TransAM NF-kB Flexi kit?
Not all NF-kB sites are the same and our Flexi kits allow users to design and bind their own custom DNA oligonucleotides to the plate. This allows for the study of NF-kB dimers which bind to specific endogenous DNA sequences.
How should I prepare my nuclear extract?
Any of the following are compatible.
- Active Motif’s Nuclear Extract Kit (Cat. No. 40010/40410)**
- Preparation of Nuclear Extract protocol in the Appendix of the TransAM manual.
- Your own nuclear extract protocol, as long as the lysis buffers do not contain SDS. Be sure to resuspend the nuclear pellet in Complete Lysis Buffer.
** Note that some TransAM kits contain Lysis Buffer AM1 while others contain Lysis Buffer AM2. If working with a TransAM kit containing Lysis Buffer AM1, use the Nuclear Extract Kit as written. If working with a TransAM kit containing Lysis Buffer AM2, prepare the Complete Lysis Buffer in the Nuclear Extract Kit using Lysis Buffer AM2 rather than the AM1 that comes in the kit. If you have already prepared your samples using the Lysis Buffer AM1 from the Nuclear Extract Kit, we recommend that you dilute these 1:3 with the Lysis Buffer AM2 that came with your TransAM assay.
The TransAM manual contains instructions for the preparation of nuclear extract from cultured cells. Do you have a protocol for tissue?
The Active Motif Nuclear Extract Kit (Cat. No. 40010/40410) contains a protocol for creating a nuclear extract from tissue. You can also use your own tissue nuclear extract protocol as long as you resuspend the nuclear pellet in the Complete Lysis Buffer recommended in the TransAM kit manual. Avoid using SDS as it can interfere with the assay.
For the nuclear extraction what should I see under the microscope when I monitor cell lysis?
Under a phase contrast microscope intact cells should appear as a dark central region (nucleus) surrounded by a halo of less dense cytoplasm. In lysed cells, the nuclei will appear as dots surrounded by asymmetric debris. See example below:
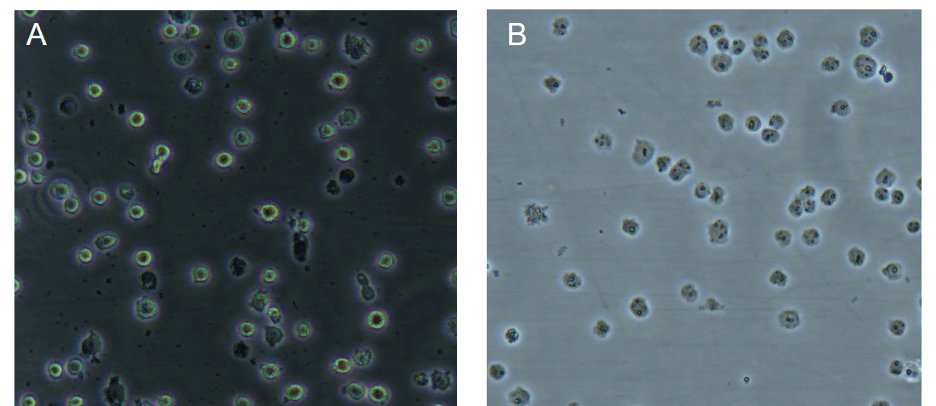
Figure: Images under phase contrast microscope of Jurkat cells before (A) and after (B) cell lysis; published with permission from Ziwei Wang, Tsinghua University.
Do you have any recommendations for nuclear extraction from PBMCs?
PBMCs are more difficult to lyse and can be resistant to lysis by the Hypotonic Buffer. Verify cell lysis efficiency using phase-contract microscopy. If cells are not adequately lysed, use a dounce homogenizer with a small clearance pestle (0.025–0.076 mm) to homogenize the sample or draw the sample through a 28-30 gauge needle several times for mechanical shearing. Active Motif’s Dounce Homogenizers for 1 mL capacity (Cat. No. 40401) and 15 mL capacity (Cat. No. 40415) both come with a small and large clearance pestle.
Do you have any recommendations for nuclear extraction from primary cells?
Primary cells can be challenging because they are typically fragile and can easily lyse with just scraping. Also, the number of cells is often limited. Be sure to monitor cell lysis under the microscope to maximize the extraction yield.
What dounce homogenizer should I use for the nuclear prep?
For cells, we recommend a dounce homogenizer with a small pestle clearance (0.025–0.076 mm) to lyse the cells and release the nuclei. For tissue we recommend a dounce homogenizer with a large pestle clearance (0.089–0.14 mm) to first homogenize the tissue into a single cell slurry and then a dounce homogenizer with a small pestle clearance to lyse the cells and release the nuclei. Active Motif’s Dounce Homogenizers for 1 mL capacity (Cat. No. 40401) and 15 mL capacity (Cat. No. 40415) both come with a small and large clearance pestle.

How many times can I freeze-thaw my nuclear extract?
It’s best to aliquot the extracts so that freeze-thaw cycles are not required. However, if you must thaw, we do not recommend freeze-thawing more than 2 times. Multiple freeze-thaws degrade proteins and destroy protein activity. Thaw the extracts on ice using pipetting to mix. Do you not vortex protein samples vigorously.
Can I use frozen samples to create the nuclear extracts?
We recommend using fresh cells or tissue, as the freezing process can disrupt the cytoplasmic and nuclear compartments, making it difficult to get pure fractions. However, if necessary, snap freeze tissues at -80°C. Cryopreserve cells before freezing at -80°C.
I am preparing a nuclear extract. The nuclear pellet is viscous and is not resuspending well in the Complete Lysis Buffer. How can I get it into solution?
To enhance the solubility of the pellet:
- Pipet up and down a few times with a large bore pipette tip.
- Use a dounce homogenizer.
- Vortex on high for 10 seconds.
- Increase the 30-minute incubation on the rocking platform to an hour.
If needed add 5-10 ul of Complete Lysis Buffer. Be sure not to overdilute the nuclear sample. Note that certain cell types are more likely to produce a viscous nuclear pellet and yet still give good protein yields, even if it doesn’t completely disappear. Do not add detergent as it could interfere with the TransAM assay.
Can I use whole cell extract?
It depends. With a whole-cell extract, the transcription factor of interest will be diluted into a larger volume than in a nuclear extract. So, more protein (2x or more) will be required. Also, if the transcription factor is regulated by nuclear-cytoplasmic localization, using whole-cell extract could affect the functional aspect of the assay.
Can I use the TransAM kit with cytoplasmic extracts?
It is not recommended for several reasons. The TransAM kit is designed to measure protein-DNA binding, which exclusively occurs in the nucleus. Any signal with the cytoplasmic extract may not be physiologically relevant. Also, the cytoplasmic extract is going to be much more dilute as compared to nuclear extract and will contain detergents unlike the nuclear fraction that that may interfere with the assay.
How should I quantify my nuclear extract protein?
We recommend using a Bradford Assay to quantify the protein rather than BCA. A BCA assay requires a higher dilution factor to eliminate confounding interfering agents and can limit the detection of protein in all but very concentrated samples. If using the Nuclear Extract Kit (Cat. No. 40010/40410), we recommend a dilution factor of 1:50 in water. There is a protocol for creating a BSA standard curve in the Appendix of the Nuclear Extract Kit manual.
How many cells/tissue should I use for the nuclear extract?
We suggest isolating nuclear extracts from samples of approximately 8.8 x106 cells or 45 mg tissue. A tissue conversion factor for general estimation is 2x105 cells/mg.
What protein yield can I expect from 8.8 x 106 cells?
This will depend on your cell or tissue type, but in general you should expect 150-250 µg at ~3-5 mg/ml from 8.8 x 106 cells.
What components can interfere with the TransAM assays?
SDS or sodium azide in your cell lysis buffer will inhibit the binding and can render your sample incompatible.
TransAM Flexi Kit Documents
You might also be interested in:
- TransAM® NF-κB Activation Assays | Colorimetric Kits
- TransAM® NFκB Chemi Kits
- FACE™ NFκB p65 Profiler
* Technology covered under EAT-filed patents and licensed to Active Motif. Use of TransAM in NFκB-related drug discovery may be covered under U.S. Patent No. 6,150,090 and require a license from Ariad Pharmaceuticals (Cambridge, MA, USA).