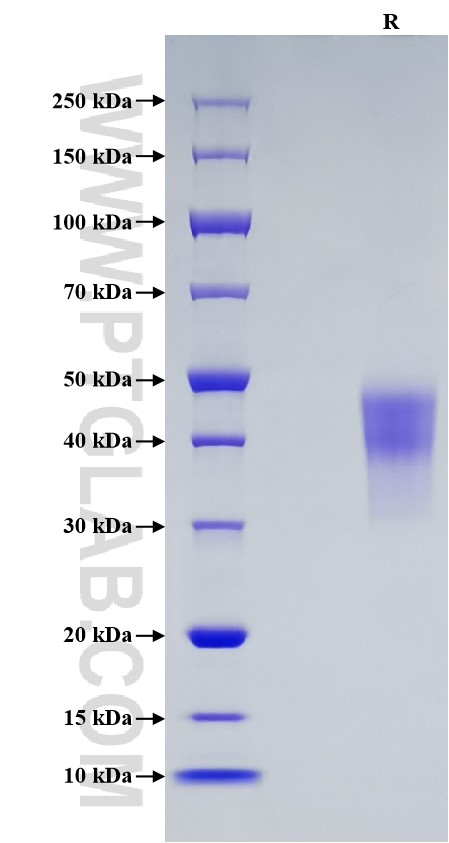
Recombinant Human OX40/TNFRSF4 protein (Myc Tag, His Tag)
ED50
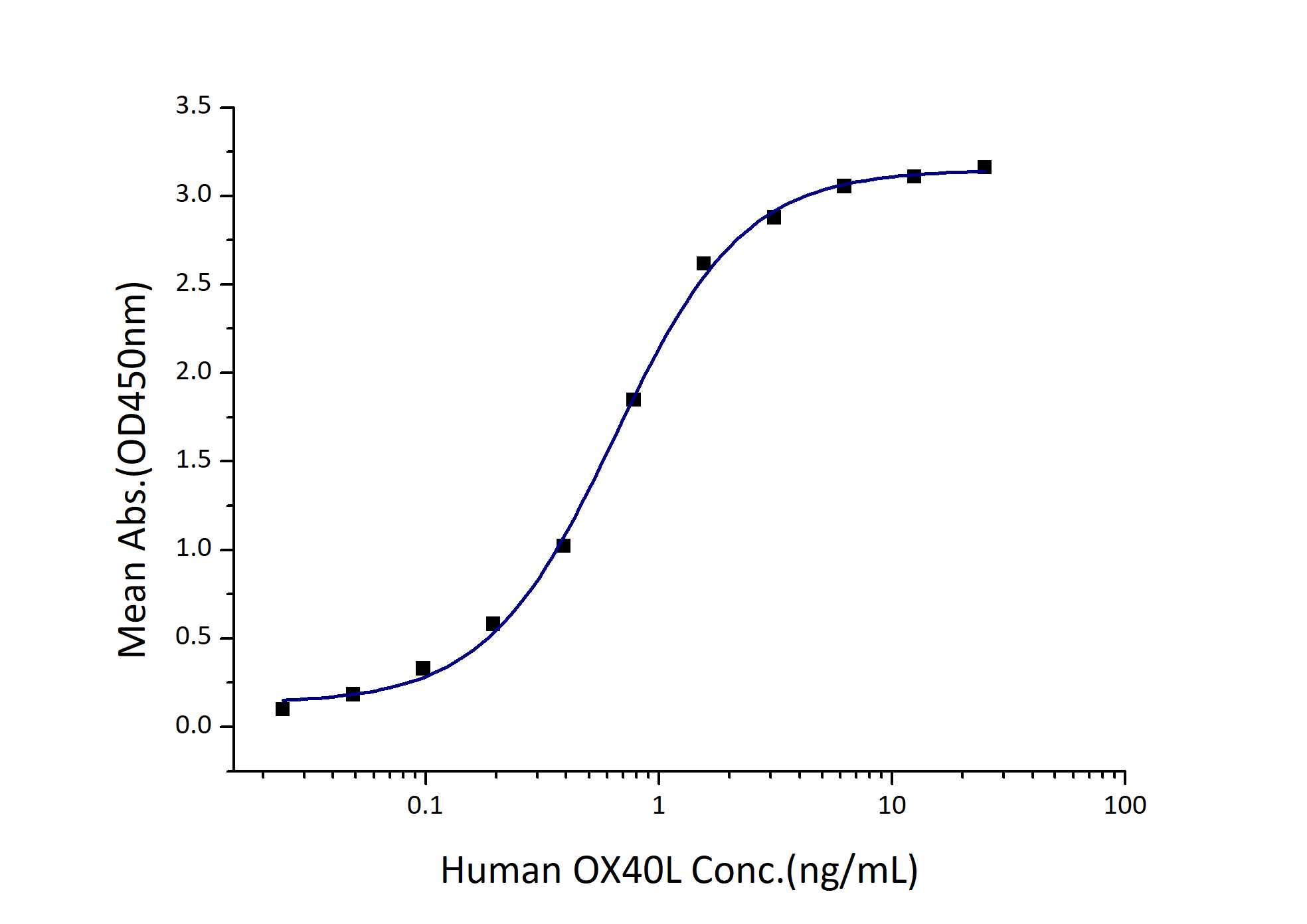
0.3-1.2 ng/mL
Species
Human
Purity
>95 %, SDS-PAGE
GeneID
7293
Accession
P43489
验证数据展示
Technical Specifications
| Purity | >95 %, SDS-PAGE |
| Endotoxin Level | <1.0 EU/μg protein, LAL method |
| Biological Activity |
Immobilized Human OX40 (Myc tag, His tag) at 2 μg/mL (100 μL/well) can bind Human OX40L (hFc tag) with a linear range of 0.3-1.2 ng/mL. |
| Source | HEK293-derived Human OX40 protein Leu29-Ala214 (Accession# P43489) with a Myc tag and a His tag at the C-terminus. |
| Predicted Molecular Mass | 22.5 kDa |
| SDS-PAGE | 38-50 kDa, reducing (R) conditions |
| Formulation | Lyophilized from sterile PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
CD134 (OX40, TNFRSF4) is a member of the TNFR-superfamily of receptors. Recent evidence indicates that OX40-OX40L coupling is critical for cell survival and proliferation. While OX40 is expressed predominantly on T-lymphocytes early after antigen activation, OX40L is expressed on activated antigen presenting cells and endothelial cells within acute inflammatory environments. The tumour necrosis factor receptor OX40 (CD134) is activated by its cognate ligand OX40L (CD134L, CD252) and functions as a T cell co-stimulatory molecule. OX40 is expressed on activated T cells, and in the mouse at rest on regulatory T cells (Treg). CD134 can interact with TRAF2, TRAF3, and TRAF5. OX40 is an immune checkpoint in cancer and its presence in cancer is a good prognosis, making it a highly relevant target for the development of new immunotherapies.
References:
1.Wu LY. et al. (2020). Exp Neurol. 326:113179. 2.Gough MJ. et al. (2009). Adv Exp Med Biol. 647:94-107. 3.Webb GJ. et al. (2016). Clin Rev Allergy Immunol. 50(3):312-32. 4.Cebada J. et al. (2021). Expert Opin Ther Pat. 31(1):81-90.