Recombinant Human TNFR2/TNFRSF1B protein (Myc Tag, His Tag)
种属
Human
纯度
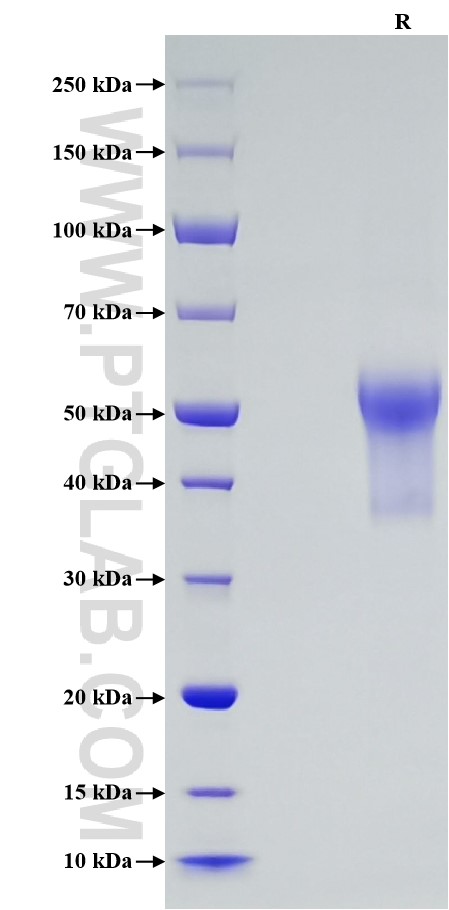
>90 %, SDS-PAGE
标签
Myc Tag, His Tag
生物活性
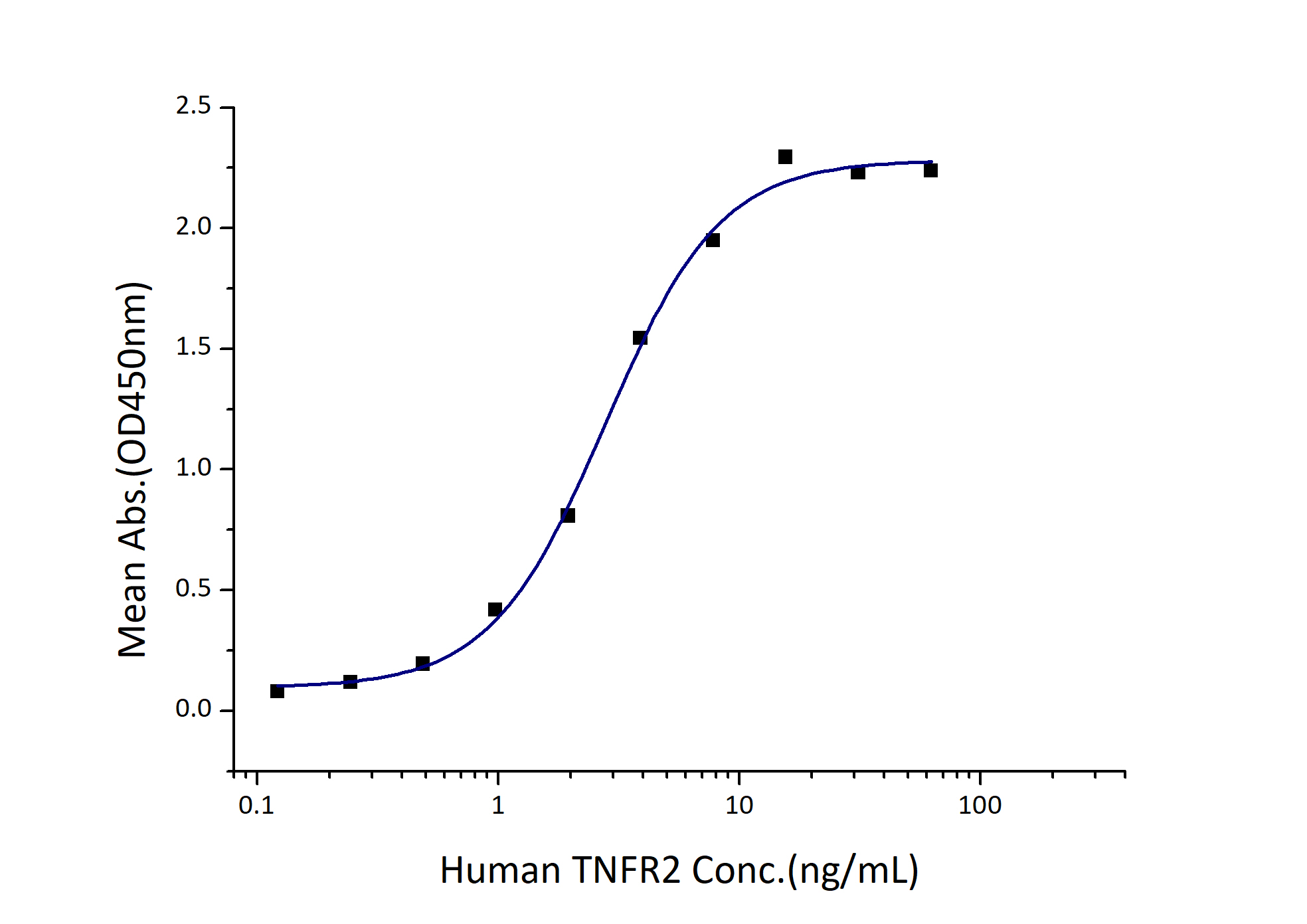
EC50: 1-5 ng/mL
验证数据展示
产品信息
| 纯度 | >90 %, SDS-PAGE |
| 内毒素 | <0.1 EU/μg protein, LAL method |
| 生物活性 |
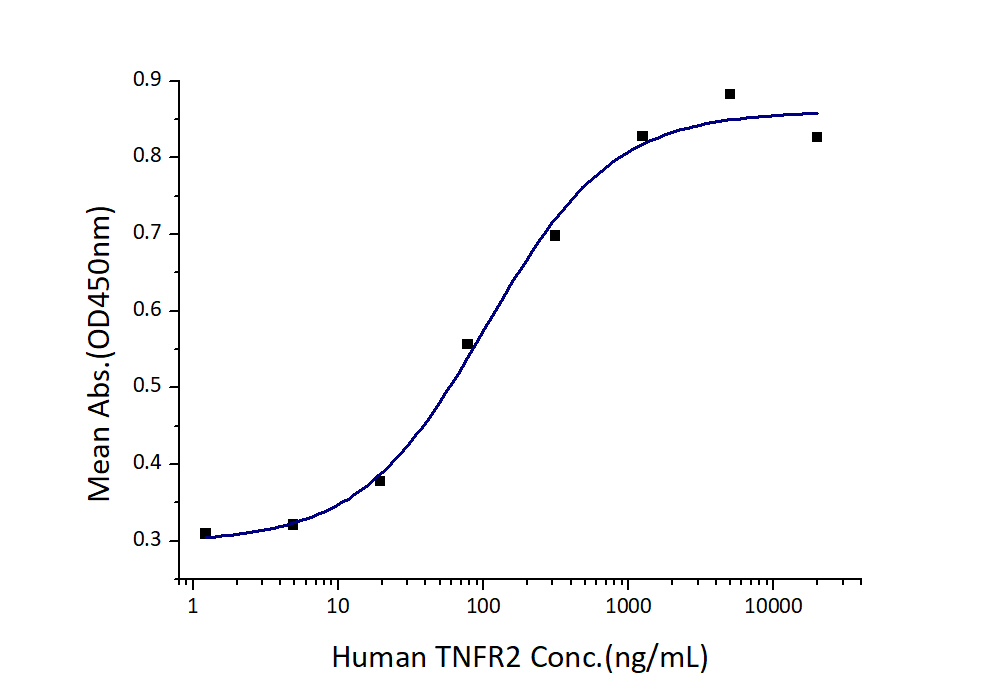
1. Immobilized Human TNF-α (GST tag) at 2 μg/mL (100 μL/well) can bind Human TNFR2 (Myc tag, His tag) with a linear range of 1-5 ng/mL. 2. Measured by its ability to inhibit TNF-α mediated cytotoxicity in the L-929 mouse fibroblast cells in the presence of the metabolic inhibitor actinomycin D. The EC50 for this effect is 50-200 ng/mL in the presence of 0.25 ng/mL recombinant human TNF-α. |
| 来源 | HEK293-derived Human TNFR2 protein Leu23-Asp257 (Accession# P20333-1) with a Myc tag and a His tag at the C-terminus. |
| 基因ID | 7133 |
| 蛋白编号 | P20333-1 |
| 预测分子量 | 30.4 kDa |
| SDS-PAGE | 48-58 kDa, reducing (R) conditions |
| 组分 | Lyophilized from 0.22 μm filtered solution in PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| 复溶 | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| 储存条件 |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| 运输条件 | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
背景信息
Tumor necrosis factor-alpha (TNFA/TNFSF2) is a multifunctional cytokine that plays a key role in regulating inflammation, immune functions, host defense, and apoptosis. TNFA signals through two distinct cell surface receptors, TNFR1 (TNFRSF1A, CD120a, p55) and TNFR2 (TNFRSF1B, CD120b, p75). TNFR2 is a kind of receptor with high affinity for TNFSF2/TNF-alpha and approximately 5-fold lower affinity for homotrimeric TNFSF1/lymphotoxin-alpha. The TRAF1/TRAF2 complex recruits the apoptotic suppressors BIRC2 and BIRC3 to TNFRSF1B/TNFR2. This receptor mediates most of the metabolic effects of TNF-alpha. In contrast to TNFR1, TNFR2 does not have a death domain. TNFR2 only signals for antiapoptotic reactions. However, recent evidence indicates that TNFR2 also signals to induce TRAF2 degradation. Various defects in the TNFR2 pathway, due to polymorphisms in the TNFR2 gene, upregulated expression of TNFR2 and TNFR2 shedding, have been implicated in the pathology of several autoimmune disorders.
参考文献:
1.Islam A. et al. (2006). J Biol Chem. 281(10):6860-73. 2.Pan S. et al. (2002). Mol Cell Biol. 22(21):7512-23. 3.Faustman D. et al. (2010). Nat Rev Drug Discov. 9(6):482-93.