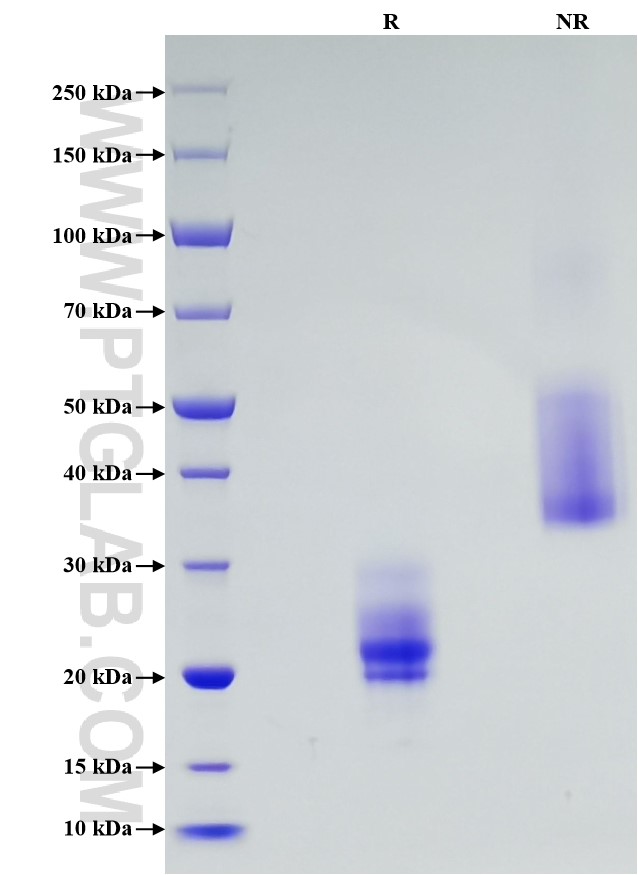
Recombinant Human VEGF121 protein (Myc tag, His tag)
ED50
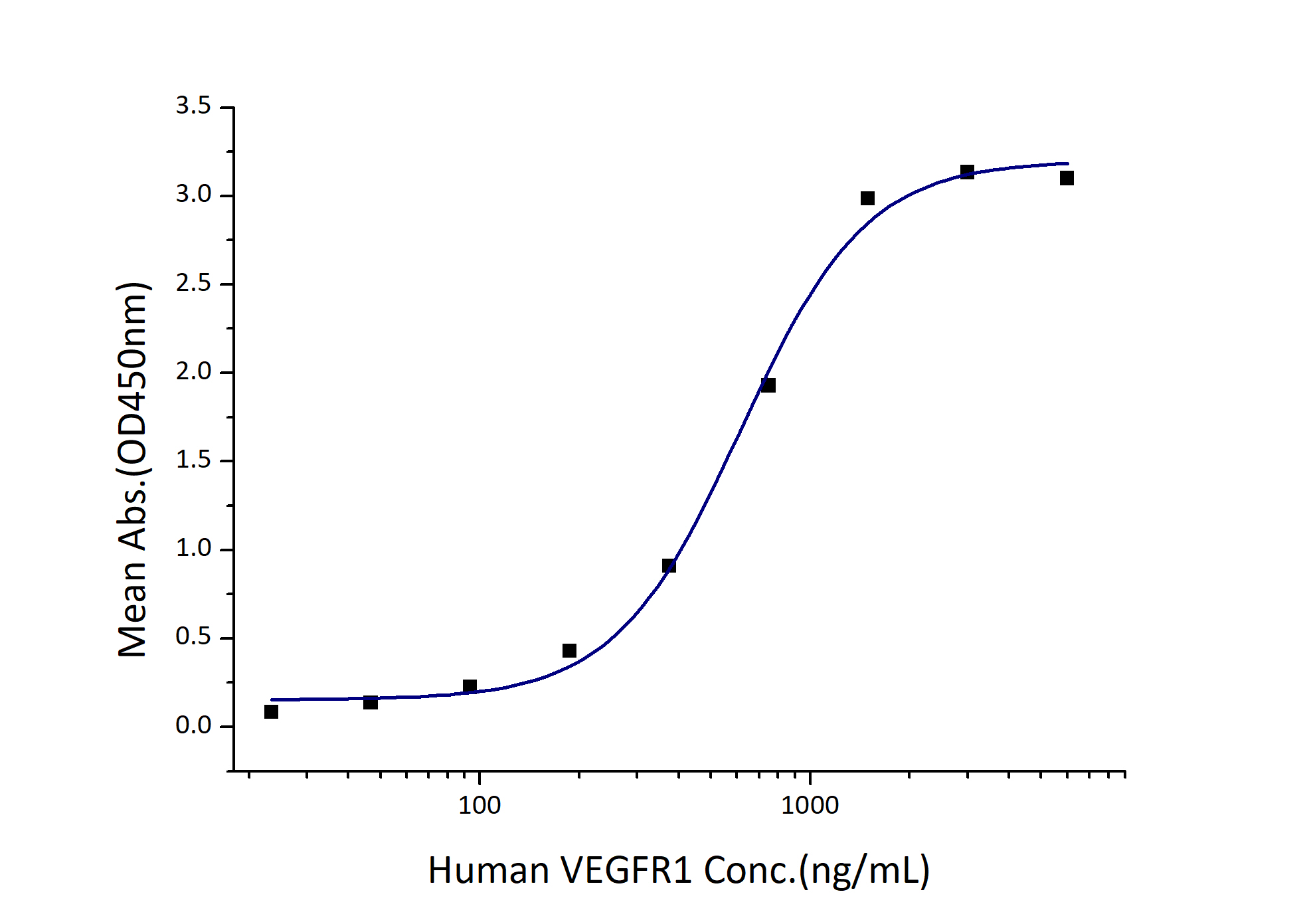
300-1230 ng/mL
Species
Human
Purity
>90 %, SDS-PAGE
GeneID
7422
Accession
P15692
验证数据展示
Technical Specifications
| Purity | >90 %, SDS-PAGE |
| Endotoxin Level | <1.0 EU/μg protein, LAL method |
| Biological Activity |
Immobilized Human VEGF121 (Myc tag, His tag) at 2 μg/mL (100 μL/well) can bind Human VEGFR1 (His tag) with a linear range of 300-1230 ng/mL. |
| Source | HEK293-derived Human VEGF121 protein Ala27-Arg147 (Accession# P15692) with a Myc tag and a His tag at the C-terminus. |
| Predicted Molecular Mass | 16.9 kDa |
| SDS-PAGE | 20-24 kDa, reducing (R) conditions |
| Formulation | Lyophilized from sterile PBS, pH 7.4. Normally 5% trehalose and 5% mannitol are added as protectants before lyophilization. |
| Reconstitution | Briefly centrifuge the tube before opening. Reconstitute at 0.1-0.5 mg/mL in sterile water. |
| Storage |
It is recommended that the protein be aliquoted for optimal storage. Avoid repeated freeze-thaw cycles.
|
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the recommended temperature. |
Background
Vascular endothelial growth factor (VEGF), is a signal protein produced by cells that stimulates vasculogenesis and angiogenesis. It is part of the system that restores the oxygen supply to tissues when blood circulation is inadequate such as in hypoxic conditions. Serum concentration of VEGF is high in bronchial asthma and diabetes mellitus. The activities of VEGF are not limited to the vascular system; VEGF plays a role in normal physiological functions such as bone formation, hematopoiesis, wound healing, and development. Disruption of this gene in mice resulted in abnormal embryonic blood vessel formation. VEGF is upregulated in many known tumors and its expression is correlated with tumor stage and progression. VEGF exists in several isoforms with different molecular weights and biological properties containing 121, 145, 165, 189, and 206 amino acids.
References:
1. Senger DR. et al. (1983). Science. 219: 983-5. 2. Ferrara N. et al. (1992). Endocr Rev. 13: 18-32. 3. Boocock CA. et al. (1995). J Natl Cancer Inst. 87: 506-516. 4. Sunderkotter C. et al. (1994). Int J Cancer. 55: 410-422. 5. T Cohen. et al. (1995) J Biol Chem. 270(19):11322-6.