Tech Tips | In search of low molecular weight proteins
Western Blot tips for low molecular weight proteins
Regular SDS-PAGE and Western blotting techniques are robust tools for investigating a broad spectrum of proteins with molecular weights (MWs) ranging from about 30 kilodaltons (kDa) up to 250 kDa. However, at extreme ends of the MW spectrum such techniques suffer from limitations: poor separation, signal reduction or even a total absence of target bands. Low MW proteins – generally any protein with a MW less than 20 kDa – are vulnerable to poor resolution and poor retention. Therefore they are challenging to detect with standard SDS-PAGE and Western blotting protocols…but they are not impossible to detect, period. Several protocol modifications can be employed when handling these MW featherweights for their retention and improved resolution at SDS-PAGE and subsequent Western blotting.
Try Tricine
The ‘standard’ polyacrylamide gels referred to above are uniform gradient glycine-Tris gel (which will simply be referred to as glycine gels). In general, glycine gels are ideal for resolving any proteins that fall within the range mentioned previously (30-250 kDas), given the total percentage of acrylamide mixture (T%) is adjusted accordingly. (Around 8% is used to obtain the best resolution of the higher end of this range, increasing up to 16%, or even 18%, for the lower end.) Acrylamide gels based on a Tris‑Tricine buffer system, however, will greatly improve your chances of ‘seeing’ your target band(s) if you are working below the 30 kDa range. Where you see a single band hovering around ambiguously near the expected MW on a glycine gel, you will see several individual, well-resolved bands at distinct MWs on a Tricine-based one. (These will be referred to as Tricine gels from this point on.) This difference is observed even when the pH and T% of the acrylamide mixture is the same within each gel (i.e. a 15% glycine gel versus a 15% Tricine gel at the usual pH values between 6.8 and 8.8).
You can find a recipe for a 15% Tricine gel in Proteintech’s Complete guide to Western Blotting
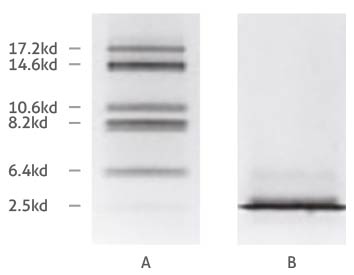
Comparison of Tricine- (A) and glycine-SDS-PAGE (B) separation of myoglobin fragments. Adapted from Schägger and von Jagow 1987.
The difference in separation capabilities of glycine and Tricine gels is attributed to the strongly differing properties of the glycine and Tricine compounds, such as their pK values and ionic mobility. We won’t go into too much detail here, but a basic explanation of why Tricine is more optimal for the separation of low MW proteins is that it ‘stacks’ proteins differently to glycine. The purpose of stacking layer is to pack proteins into uniform bands, so that groups of proteins enter the separating layer at the same time – like runners lining up at a start line before a race. (For instance, smeared bands can occur when a protein gets a head start from its protein ‘pack’.) A Tricine-based stacking layer shifts the upper stacking limit (i.e. the molecular mass of the largest protein in a given stack) down to as low as 30 kDa for the first stack1. Because proteins above the 30 kDa mark are already separated from the stack of sub-30 kDa proteins before reaching the separating layer, it prevents overloading at the interface between the gel layers, giving the stack containing the smallest proteins the best start to separationref1. The greater ionic mobility of Tricine also means that lower percentages of acrylamide can be used in gels to achieve the same degrees of separation – so higher, yet still moderate acrylamide concentrations can be used to resolve a narrow window of low MW proteins e.g. a 15% Tricine gel for the range between 5 and 20 kDa. But what about anything below 5 kDa you ask? Adding 6 M urea in the gel mixture enhances the resolution of small proteins furtherref2, which is recommended for anything lower than 5 kDa.
Protein transfer
As well as ensuring the optimum separation of low MW proteins, you also need to take care at the protein transfer stage. Low MW proteins are susceptible to ‘over transfer’ – loss of sample due to lack of retention by the transfer membrane and/or too rapid transfer.
Membrane choice and pore size
Most labs have a preferred choice of Western blotting membrane, be it nitrocellulose or PVDF (polyvinylidene fluoride), but PVDF is a better choice in the case of smaller, low MW proteins. It has a greater capacity to bind proteins compared to its nitrocellulose counterpart. PVDF has a protein binding capacity of 170 to 200 μg/cm2 while nitrocellulose has a protein binding capacity of 80 to 100 μg/cm2 ref3. This article discusses a few more of the considerations you should take into account when choosing a membrane for Western blotting, complete with images of the microstructure of both PVDF and nitrocellulose membranes (which is part of the explanation protein retention properties of PVDF are slightly higher).
Whichever membrane you choose, be aware there are various pore sizes on offer; both are available as 0.45 μm, 0.2 μm, or 0.1 μm versions. Opt for a smaller pore sized version to obtain better transfers of your low MW target proteins – a 0.2 μm membrane is more than adequate for use with any proteins weighing in less than 20 kDa. The Proteintech lab uses Millipore’s Immobilon PSQ PVDF membrane for the transfer of low MW proteins.
Transfer conditions
In addition to membrane choice, other factors like transfer system, length, temperature and buffer composition come into play when dealing with low MW proteins. Semi-dry transfer systems seem to have the edge over wet transfer apparatus in this arena (probably due to the simple fact that semi-dry systems are less vulnerable to over transfer because of their efficient transfer times). Be aware however, that semi-dry transfer systems can suffer from reproducibility issues (but this should be less so with low MW targets).
In the case of small proteins, less is often more when it comes to transfer length and voltage (or amperage depending on your setup). How much you should adjust these by will be down to the brand and model of system being used. Look up the recommended transfer times and voltages for proteins below <30 kDa in the equipment manual – if one of these isn’t available, you should still be able to find this information on the manufacturer’s website.
Other tips
You may be using bromophenol blue in the sample loading buffer when running samples on a gel, but be aware some proteins will escape the dye front!
You can choose to soak your gel in an SDS-free buffer (or simply good old H2O) for 5 minutes prior to setting up your transfer. This helps to remove SDS, which coats small proteins and protein fragments in negative charges, increasing their rate of passage through the Western blot membrane.
Happy hunting!
References
1. H Schägger and G von Jagow. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166(2):368-79.
2. H Schägger, Tricine-SDS-PAGE, Nature Protoc. 2006;1(1):16-22.
3. The Protein Man’s Blog, PVDF or Nitrocellulose – Which Membrane is Best? Nov 12, 2014:http://info.gbiosciences.com/blog/bid/203026/PVDF-or-Nitrocellulose-Which-Membrane-is-Best





