Detecting low abundance proteins via Western Blot
Detecting low abundance proteins can be a challenge - here's how to optimise your Western Blot.
Written by Kathryn Green, PhD student at The University of Nottingham
Why is there a need for higher sensitivity western blots?
Western blotting is a prominent analytical technique used to study the expression and abundance of a particular protein within a sample. It can provide a great deal of information and consequently is a powerful tool in many research settings. Despite this, detection of some low abundance proteins has proven to be tricky. Fortunately, there are plenty of steps you can take to increase the likelihood of observing a low abundance protein on a western blot.
There are multiple reasons why a researcher may need to detect low abundance proteins. Not only do some proteins have low expression, but they can also be difficult to extract due to their location. Sometimes the nature of the assay may result in a low total protein concentration, and in many instances the sample amount itself is limited, such as the use of rare tissue samples. All these considerations, along with the fact multiple assays are often required from one condition, mean the amount of sample available for western blot is frequently limited. Luckily there are multiple ways in which the western blot protocol can be optimized to detect these low abundance proteins.
Higher sensitivity detection methods
There are a variety of western blot detection methods to utilize, each with its pros and cons. One of the most common WB detection methods is achieved by using antibodies conjugated to the enzyme horseradish peroxidase (HRP), which reacts with luminol and peroxide buffer to produce light. Using antibodies conjugated to a fluorophore is also a popular alternative technique since this allows for simple and easy multiplexing. However, a drawback of fluorescent detection is the system is less sensitive in comparison to chemiluminescent detection. Because of this, it is highly recommended to use HRP and chemiluminescence when detecting these low abundant proteins.
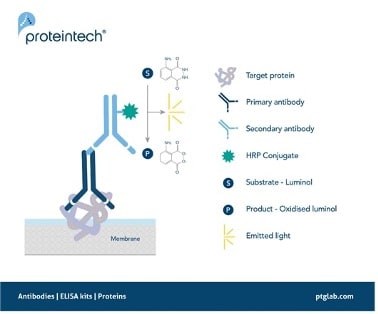
Figure 1. Signal generation in chemiluminescent western blotting.
A secondary antibody with an HRP conjugate enables the detection of a target protein by oxidizing its substrate luminol with the emission of light as a by-product.
Enhanced Chemiluminescent Substrate
Low abundant proteins can still be challenging to detect with standard chemiluminescent systems due to low signal sensitivity and/or low signal-to-noise ratio. A highly effective way of increasing sensitivity is by using enhanced sensitivity substrates. These are designed to produce higher levels of chemiluminescence signal compared to your standard substrates. Using a substrate that provides a brighter signal will therefore increase the chance of visualizing these low abundance proteins.
Proteintech has generated SignalBright, a range of enhanced chemiluminescent substrates capable of detecting femtogram levels of protein. Using SignalBright increases the chance of generating adequate signals by providing ultra-sensitive detection and a greater signal-to-noise ratio. This allows for the visualization of proteins in small sample volumes, or which are low in abundance. There are three different SignalBright substrates available, each with a different level of sensitivity. These are SignalBright Pro (PK10011), SignalBright Plus (PK10012), and SignalBright Max (PK10013). The three different levels of sensitivity ensure the ECL substrate is well suited to the needs of the experiment taking place. The table below summarizes each SignalBright product individually, along with important aspects of their performance.
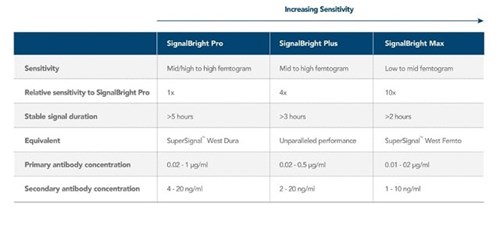
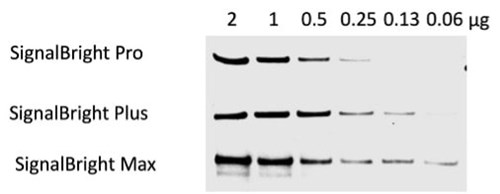
Figure 2. Comparison of Proteintech’s SignalBright Pro (PK10011), SignalBright Plus (PK10012), and SignalBright Max (PK10013) Enhanced Chemiluminescent Substrate. Serial dilutions of HeLa cell lysate. Primary antibody: Proteintech Beta Catenin 51067-2-AP, 1:20,000. Secondary: Quanta BioDesign HRP-Goat anti-Rabbit IgG (H&L) (11-0201-0503) at a 1:100,000 dilution. Exposure Time: 30 seconds.
SignalBright ECL substrate also has the benefit of providing a bright and stable signal for over 5 hours. Therefore, the requirement for quick access to an imager is removed. It also means adjustments can be made whilst imaging to ensure you capture the signal exactly as you wish, without having to worry about losing any signal.
Efficient extraction and total transfer
Ensuring there is efficient extraction of the protein of interest is one step you can take right at the start of your western blot protocol. Some proteins are particularly hard to extract due to their location, for example proteins that are commonly located in the nucleus such as transcription factors or histone modifiers. These proteins rely on efficient lysing of not only the cell membrane but also the nuclear envelope to release these proteins into the lysate. The same can also be said for other localized proteins such as those which reside in the mitochondria. Using a lysis buffer such as RIPA is therefore recommended as it contains SDS, a harsher detergent to ensure complete lysis of these intracellular compartments. Alternatively, using fractionation kits to extract specific parts of the cell e.g. mitochondria or nucleus can help enrich your sample, increasing extraction efficiency of your protein of interest.
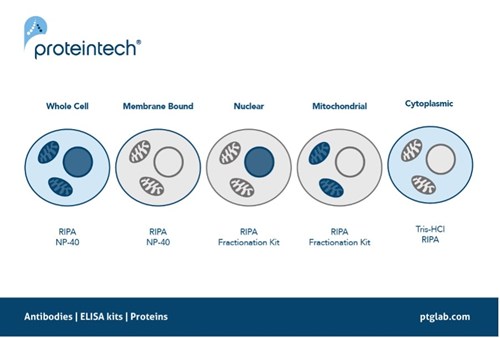
Figure 4. The recommended protein extraction buffers depending on the localization of your protein of interest.
Finally, ensuring there has been a total transfer of protein onto your membrane before probing is another critical step. If your target is of low abundance, any loss in the transfer step may mean a total loss of your signal. There are multiple transfer methods, each with varying specifications. Optimizing the transfer of your protein of interest is highly suggested. For example, higher molecular weight proteins may require longer transfer times and at higher voltages. We also recommend using PVDF membrane as it has a higher binding capacity in comparison to nitrocellulose. Not only this, but it also has less non-specific binding of the antibodies to the membrane, producing less background.
Antibody validation and considerations
Optimizing your antibody concentration is even more important when using high-sensitivity ECL substrates. A common misconception is that increasing the concentration of the primary and secondary antibodies will lead to a better signal. However, this is not always the case, especially when using high-sensitivity ECL since too much secondary antibody can lead to high background and signal variability due to overloading the HRP-luminol causing increased enzyme activity. It is recommended that when using high-sensitivity ECL, the primary and secondary antibody concentrations are dramatically decreased, and to always follow manufacturer recommended antibody concentrations. Not following recommended concentrations can lead to totally black and ruined blots. Using highly sensitive ECL can require some optimization however, all our SignalBright substrates come with recommended concentrations and dilutions to aid this optimization process.
The importance of using validated antibodies for your target is evident in all antibody utilizing techniques, and western blotting is no different. Ensuring your primary antibody is validated and has proven to produce reliable data is one of the essential steps in your search for low abundance proteins. Using a knockdown/knockout validated antibody is ideally what you want to be using in your research, as this is the most definitive way of ensuring specificity. These antibodies have been confirmed to be specific for their target by either knocking down or knocking out the expression of the protein and then ensuring the signal is diminished. Proteintech has the largest range of KD/KO validated antibody in the antibody industry, making them an excellent choice to pair with high-sensitivity ECL substrates.

Figure 3. Knock-down validation result of SIRT1 antibody (13161-1-AP). Significant decrease in signal in SIRT1 siRNA treated HEK-293 cells.
Summary
Although low abundance proteins are sometimes difficult to observe via western blotting, it is certainly not an impossible task. Using ECL substrates with higher sensitivities is a worthwhile way to improve the likelihood of observing your low abundant protein on a WB. This, along with the various steps highlighted above, should put you on the right path when searching for those precious bands!


