Polyclonal vs. monoclonal antibodies
Both types have their unique advantages and disadvantages and can be used in a wide variety of applications.
Introduction
Antibodies are large Y-shaped proteins called immunoglobulins which are produced by B cells as part of the adaptive immune response when encountering a foreign molecule. Due the strong affinity of an antibody to one particular sequence, an epitope (typically 4-6 amino acids in length), they are widely used in research to identify and detect target proteins of interest in a variety of different applications. Of the available antibody isotypes, IgG is most commonly used for research. To answer different research needs, there are two types of antibodies available to scientists: polyclonal and monoclonal. Polyclonal antibodies contain a heterologous mixture of IgGs against the whole antigen, whereas monoclonal antibodies are composed of a single IgG against one epitope (Figure 1.)
This blog aims to give a comprehensive overview of the advantages and disadvantages of these two types of antibodies to enable the user to best choose the type most suitable for their application.
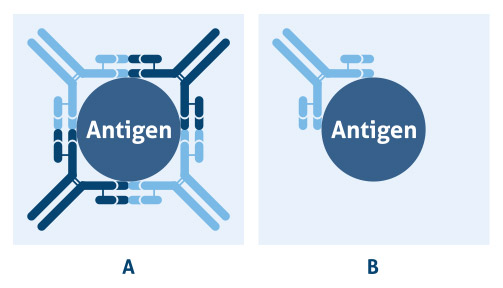
Figure 1. A) Polyclonal antibodies bind to the same antigen, but different epitopes; and B) monoclonal antibodies bind to the same epitope on a target antigen.
Polyclonal vs. monoclonal antibodies
This summary table highlights the five main differences between the two types of antibodies.
| Refer to a mixture of immunoglobulin molecules that are secreted against a particular antigen. | Refer to a homogenous population of antibodies that are produced by a single clone of plasma B cells. |
| Produced by different clones of plasma B cells. | Produced by the same clone of plasma B cells. |
| Production does not require hybridoma cell lines. | Production requires hybridoma cell lines. |
| A heterogeneous antibody population. | A homogenous antibody population. |
| Interact with different epitopes on the same antigen. | Interact with a particular epitope on the antigen. |
Polyclonal antibodies: Advantages and disadvantages
Advantages:
-
Inexpensive and relatively quick to produce (+/- 3 months).
-
Higher overall antibody affinity against the antigen due to the recognition of multiple epitopes.
-
Have a high sensitivity for detecting low-quantity proteins.
-
High ability to capture the target protein (recommended as the capture antibody in a sandwich ELISA).
-
Antibody affinity results in quicker binding to the target antigen (recommended for assays that require quick capture of the protein; e.g., IP or ChIP).
-
Superior for use in detecting a native protein.
-
Easy to couple with antibody labels and rather unlikely to affect binding capability.
Disadvantages:
-
Batch-to-batch variability as produced in different animals at different times.
-
High chance of cross-reactivity due to a recognition of multiple epitopes (affinity purified antibodies display a minimum cross-reactivity).
Monoclonal antibodies: Advantages and disadvantages
Advantages:
-
Batch-to-batch reproducibility (high homogeneity).
-
Possibility to produce large quantities of identical antibody (an advantage for diagnostic manufacturing and therapeutic drug development).
-
High specificity to a single epitope reflected in low cross-reactivity.
-
More sensitive in assays requiring quantification of the protein levels.
-
Low background noise.
Disadvantages:
-
More expensive to produce. It is necessary to produce a pool of several monoclonal antibodies.
-
Requires significantly more time to produce and develop the hybridized clone (+/- 6 months).
-
More susceptible to binding changes when labeled (e.g. with a chromogen or a fluorophore).
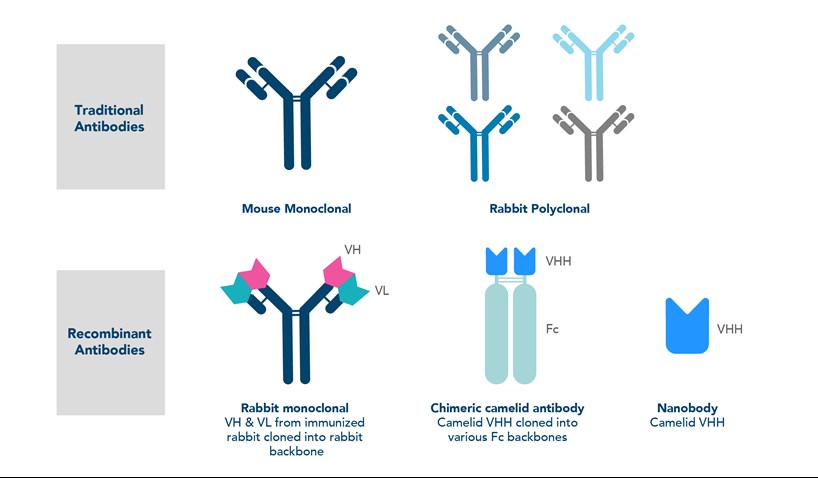
Recombinant antibodies
The next generation of monoclonal antibodies are recombinant antibodies, which are considered to be the future of antibody manufacturing. Recombinant antibodies are monoclonal antibodies that have been produced by in vitro cloning of the antibody heavy and light chain DNA sequences from the B cells or plasma cells of immunized animals. Unlike traditional monoclonal antibodies produced from hybridomas, the recombinant vectors are introduced into expression hosts (e.g. E. coli) to then produce the antibodies. This recombinant technology results in almost no lot-to-lot variability, removing the risk of genetic drift that can result in variations in of monoclonal antibodies.
Proteintech has a wide range of recombinant antibodies, and we are constantly adding new targets every month. Find out more about our recombinant antibodies and production process here.
Final remarks
Polyclonal antibodies are made using several different immune cells. They will have the affinity for the same antigen but different epitopes, while monoclonal antibodies are made using identical immune cells that are all clones of a specific parent cell.
For applications such as therapeutic drug development that require large volumes of identical antibody specific to a single epitope, monoclonal antibodies are a better solution. For general research applications, however, the advantages of polyclonal antibodies typically outweigh the few advantages that monoclonal antibodies provide. With affinity purification of serum against small antigen targets, the advantages of polyclonal antibodies are further extended.




