FGF Basic TS
Thermostable FGF does not require media changes over the weekend.
FGF Basic background
Basic fibroblast growth factor (FGFbasic), also known as bFGF, FGF2, FGF-β, or HBGF-2, belongs to the FGF family.
-
FGF plays important roles in diverse biological functions in vivo and in vitro.
-
FGF is involved in embryonic development, neuron differentiation, and the proliferation of cells of mesodermal origin and many cells of neuroectodermal, ectodermal, and endodermal origin1,2.
Proteintech has developed a thermostable FGF (FGFbasic-TS)
| Animal-free Recombinant Human FGFBasic-TS | |
FGF is a required component of stem cell culture media for maintaining cells in an undifferentiated state. Because FGF is unstable, daily media changes are needed.
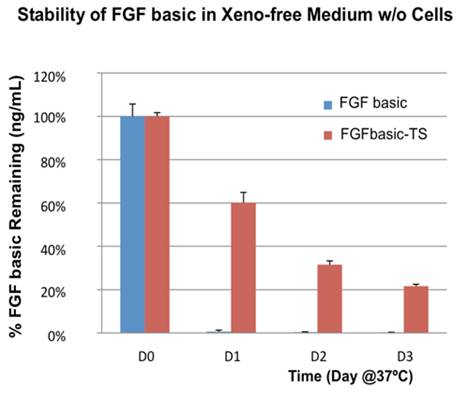
Proteintech has developed a thermostable FGF basic (FGFbasic-TS) that supports a 2-day media change schedule, so no media changes are required over a weekend (Figure 1).
Proteintech FGFbasic-TS is created in HEK293 cells using animal-free components
FGFbasic is essential for stem cell culture3. Current offerings in the market are made using bacteria or animal cells, which raises the risk of pathogen transmission4.
-
FGFbasic-TS was engineered for enhanced stability in culture media, without modification of its biological function.
-
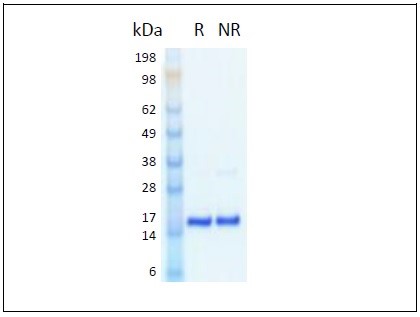
The 154 amino acid, 17 kDa, non-glycosylated monomer cytokine is expressed in Proteintech’s proprietary human cell line (HEK293) developed for the production of authentic human recombinant proteins.
-
FGFbasic-TS has greater stability than E. coli-derived FGFbasic, lasting 3X longer at 37˚C (Figure 2).
Biochemical and Cell Culture Analysis of FGFbasic-TS
Pluripotency Markers: SSEA-1, Tra 1-60, Tra1-81, Oct3/4
-
Molecular markers are one method to characterize the status of a pluripotent stem cell by their expression over passaging.
-
FGFbasic-TS was evaluated for effective maintenance of pluripotency markers using a 2-day feeding schedule in human embryonic stem cells (hESCs) and human-induced pluripotent stem cells (iPSCs).
Protocol overview:
-
FGFbasic-TS was added at 10 ng/mL.
-
Human cell lines were cultured in xeno-free, chemically defined media containing either a xeno-free chemically defined matrix (XFM) or Matrigel (MG).
-
Cells were passaged using either xeno-free, non-enzymatic passaging solution (XFPS) or a collagen-based reagent (CG).
-
Pluripotency cell surface markers were analyzed by flow cytometry for hESC and iPSC cultures grown in media containing 7.5 or 10 ng/mL FGFbasic-TS on the starting culture and after the 10th split, for all culture conditions (Figure 3 and 4).
| Figure 3 |
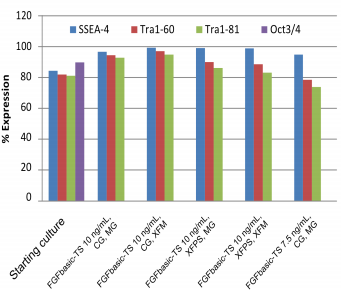 |
| Pluripotency markers in hESC cultures maintained with thermostable FGF basic, in the starter culture and after 10 passages. |
| Figure 4 |
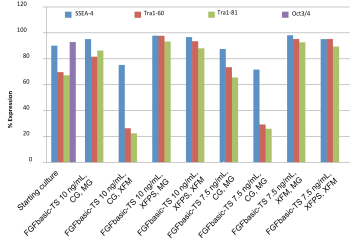 |
| Pluripotency markers in iPSC cultures maintained with thermostable FGF basic, in the starter culture and after 10 passages. |
Resistance to Enzymatic Digestion
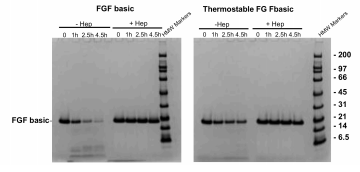
FGF basic and FGFbasic-TS were subjected to a tryptic digest then analyzed on an SDS-PAGE gel, without and with Heparin to stabilize against degradation by trypsin.
-
FGF basic was significantly degraded after 4.5 hours, while FGFbasic-TS protein levels were only slightly reduced (Figure 5).
-
FGFbasic-TS was more stable in cell culture media and more resistant to proteolytic degradation compared to FGF basic.
Proteintech FGFbasic-TS HZ1285 maintains cell growth, pluripotency, and differentiation potential with a 2-day feeding schedule.
- Broad applicability for stem cell culture - can be used for multipotent or oligopotent cell types before they differentiate into mature effector cell types and also for Mesenchymal Stem Cells (MSCs), Neuronal Stem Cells (NSCs), and Hematopoietic Stem Cells (HSCs)
-
Thermostable - maintains cell cultures more efficiently and homogeneously
-
Cost-effective - requires less frequent media changes for cell culture
-
High biological activity - can retain its biological activity for over 2-3 days at 37°C
Related resources
-
Benefits of HumanKine® recombinant proteins for clinical applications
-
Recombinant human protein Activin A: An Alpha in the TGF Beta Family
- GM-CSF – A modulatory cytokine in autoimmunity & inflammation
- Stemple D, Mahanthappa N, Anderson D. Basic FGF induces neuronal differentiation, cell division, and NGF dependence in chromaffin cells: A sequence of events in sympathetic development. Neuron. 1988;1(6):517-525.
- Rydel R, Greene L. Acidic and basic fibroblast growth factors promote stable neurite outgrowth and neuronal differentiation in cultures of PC12 cells. The Journal of Neuroscience. 1987;7(11):3639-3653.
- Xu R, Peck R, Li D, Feng X, Ludwig T, Thomson J. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nature Methods. 2005;2(3):185-190.
- Pera M. Stem cell culture, one step at a time. Nature Methods. 2005;2(3):164-165.