Why Human Cell Expressed Proteins Are Superior for Clinical Applications
For Humans...By Human Cells
Why Human Cell Expressed Proteins Are Superior for Clinical Applications
Breakthroughs in molecular biology during the 20th century to write, edit, and erase DNA created new possibilities for medicine. A successful realization of this potential is the use of a tractable living system to make an active human protein. A notable early example was the use of E. coli by Genentech to make recombinant insulin (1).
As biology and medicine have changed, so too have the needs of recombinant proteins. For activity, many proteins require glycosylation and processing that is only available in eukaryotic systems. This led to the development of insect and Chinese hamster ovary (CHO) cells as recombinant protein producers.
As science and medicine have progressed, these systems have struggled to satisfy the growing number of needs. For example, human stem cell technology and CAR-T cell therapy require culture media whose components must be animal component and xenobiotic free, yet retain high activity and scalability.
For human applications and research, a human cell expression system is ideal. All our HumanKine® recombinant proteins are made using HEK293 cells and are perfect for the new needs of clinical research.
Here's why HumanKine® products are a cut above the rest:
1. Animal component and xenobiotic free
Products derived from or by an animal are not used at any point during production.
Benefits of animal component free and xeno free proteins:
|
2. Tag-free
Expressed and purified without any tags.
Benefits of tag free proteins:
|
3. Native folding and maturation
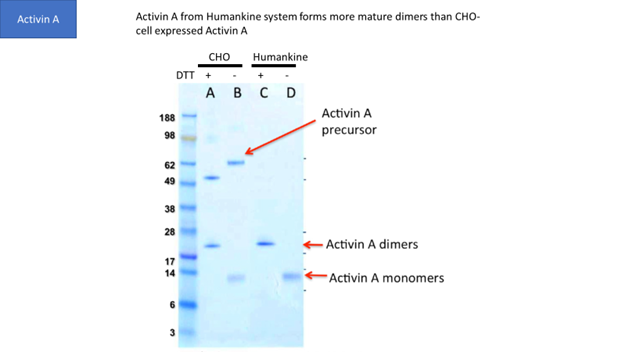
Though CHO and insect cells are eukaryotic, their ability to process human proteins does not match human cells in many cases. For example, Figure 1 shows that human cells generate more mature Activin A dimers than CHO cells.
4. Native human glycosylation
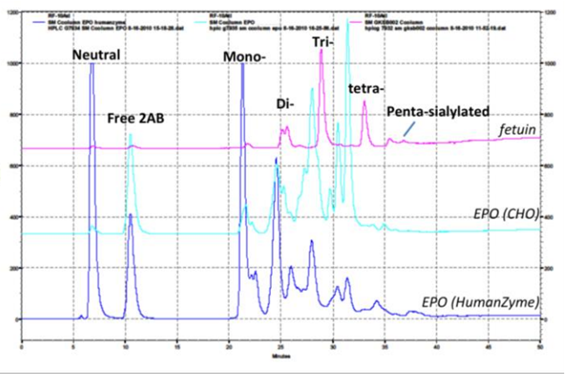
Glycosylation is crucial to stability and activity. CHO and insect cells have vastly different machinery for this process, producing glycosylated species that can be very different from humans. Figure 2 contains an example where glycosylation differs between expression systems.
5. High stability
Due to native glycosylation and maturation, human cell expressed proteins can outperform other systems in terms of stability. Figure 3 shows how HumanKine FGF has greater stability in culture than an E. coli-derived FGF.
6. High activity
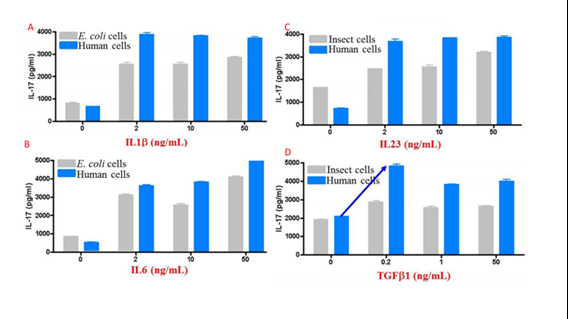
The above features synergize to create proteins that tend to have higher activity than those produced in other expression systems. Figure 4 compares activity for multiple cytokines between eukaryotic systems.
There is a common thread that unites many biotechnology stories: Discover a general principle in bacteria, then work up model organisms until ready to apply to human goals. Protein production follows this pattern and with HumanKine® products, we are finally at the stage of using human cells to make human proteins for human use.
Related resources
-
Recombinant human protein Activin A: An Alpha in the TGF Beta Family
-
GM-CSF – A modulatory cytokine in autoimmunity & inflammation
- FGF Basic TS: Thermostable FGF does not require media changes over the weekend
See below for a full list of HumanKine® products: