Cytokines and Growth Factors
Cytokines and Growth Factors - Why you should give a HEK
This webinar discusses the advantages of manufacturing our GMP-grade HumanKine cytokines and growth factors using HEK293 cells.
Video Transcription
Hi. Good afternoon, good evening to some of you, and good morning. I realize that we've got people where it's very late at night and very early in the morning, so thanks to everyone for joining us this afternoon where I am.
I'll just go over a couple of what would normally be where the fire exits are and general housekeeping before we get started and wait for everyone to join. Welcome to the next webinar in our Back to the Lab series. There are some other webinars that are available on the Proteintech website if you want to have a look at them and pass them on to any of your colleagues. We're now seeing more people getting back into the lab, so we've made a series of webinars just as little refreshers at this time when more people are starting to work from home as well as there being less people in the lab at any one time.
You will be able to see a questions tab. I'm afraid I'm the only one that has a microphone that works, so there is a questions tab there if you want to ask any questions as we go along. There is also a handout available that you'll be able to see in the list there, that you can download. You can also see my email address there, fiona@ptglab.com, if there is anything that you want to ask on a one-to-one or any downloads that you want me to send through to you. Just feel free to get in touch.
Just a little bit about me before I start talking about the cytokines and growth factors. I joined Proteintech last year following the completion of our facility in Chicago. I've got a background in regenerative medicine and have worked in commercial roles for probably longer than I wish to say, but more than 15 years, and I cover the HumanKine range for Europe. My colleague, Erika Howard, does a similar role and is based in the US. She covers North America and is the HumanKine specialist out there.
Introduction to Proteintech
Today I'm going to talk about the advantages of human expression systems for recombinant proteins and why you should give a HEK. Proteintech, just as a synopsis of the company, is an original manufacturer of antibodies, proteins, and ELISA kits. It was founded by Jason Li in 2002 as a spin-out from the University of Illinois. He really wanted better quality antibodies and if you ever meet Dr. Li, you will find out that the only way of him getting those good quality antibodies was for him to make them himself.
At a similar time, HumanZyme was also spun out of the University of Illinois, and they took the decision to only use human cell expression systems right from the start. Whereas other people were going along the line that we normally go on in biotechs where we start off in bacteria and work up to mammalian cells, they made the decision to use the information that we had and just start in human systems as we meant to go on. It became an almost natural acquisition in 2018, and Proteintech immediately focused on the GMP manufacturing of recombinant proteins at the facility in Chicago, and in the background of this slide, you can see the manufacturing facility there.
HumanKine products are recombinant proteins including the cytokines, chemokines, growth factors and interleukins. As we go on today, you will hear me refer to these as recombinant proteins or simply as proteins, but I'm talking about all of these products here, from cytokines through the interleukins. Other products that are in this range are human serum albumin, which is often used to reconstitute the lyophilized products, AuthentiKine ELISA kits, and neutralizing antibodies.
Onto the HEK293 expression system. Recombinant proteins are made in a variety of cell types, in E.coli, in Chinese hamster ovary, and also in HEK293, which is what all of the HumanKine proteins are expressed in. HumanKine proteins are expressed using stable HEK293 cell expression systems. As I say, there are alternative expression systems like mammalian ones in hamsters, bacterial and insect cells. Also, yeast can be used for recombinant proteins, but really as we develop and move away, HEK293 cells are being used more and more. This ensures that the proteins are authentic proteins and have native confirmation and post-translational modifications. They're carrier-free and, as you can see in the colored tabs in the bottom here, they have almost natural qualities of being animal component-free, endotoxin-free, xeno-free and tag-free. But I'll go on to talk about that a little bit more later.
Advantages of the HEK293 expression system is that they're authentic human proteins that are made by those human cells with optimized biological activity. Developed for the advance therapy medicinal product manufacturers and regenerative medicine marketplaces, they're manufactured in GMP conditions, and as our RUO and GMP products are manufactured using the same process and in the same facility, there is an easy transfer from using RDO in development to GMP at tech transfer.
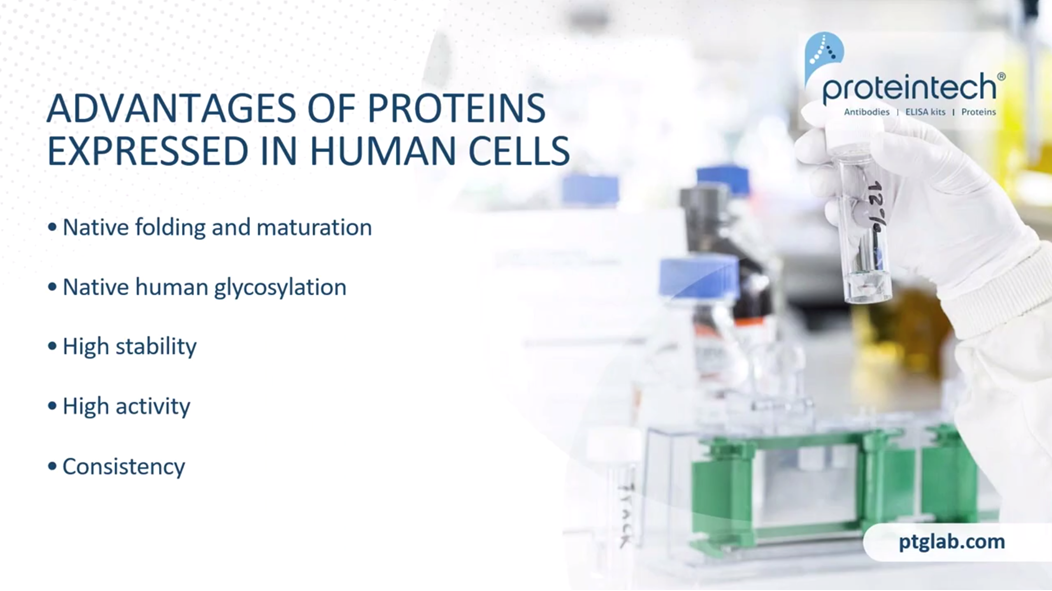
The advantages of proteins that are expressed in human cells, above and beyond them being authentic human proteins, is the native folding and maturation. Although CHO and insect cells are eukaryotic, their ability to process human proteins does not match the human cell in many cases. Native human glycosylation is crucial to stability and activity. CHO and insect cells have vastly different machinery for this process, so they produce a glycosylated species that's really quite different from humans. High stability. Due to that glycosylation and maturation, the human cell-expressed proteins can outperform other systems. In terms of stability, particularly if they are heavily glycosylated, that makes a huge difference to the stability.
All of these features synergize together to create proteins that tend to have higher activity than those that are produced in CHO and E.coli Cells, and also with consistency, there is less lot-to-lot consistency and it's really predictable, the consistency that we're going to get. In terms of yields as well, especially compared to, for example, transient cell lines.
All of these recombinant proteins are expressed and purified without tags, follow a struct animal-free and xeno-free policy. These products are produced aseptically in human cells, so they are virtually naturally endotoxin-free, although these tests are very stringent and the levels are recorded for all our data. There's minimal risk of any endotoxin levels.
The final product doesn't contain any constituent that's either an animal tissue, body fluid or a component derived from it. For example, fetal calf serum. All the materials from procurement to final products and stored and handled in dedicated animal-free facilities, and the final product to process don't involve the use of any nonhuman animal sources or recombinant materials from nonhuman animal sources either.
The inclusion of a tag can often result in changes to the structure of the protein of interest, so for example, there are no his-tags on these proteins. Sometimes a tag can interfere with the active site of the protein, so it's an altered biological activity, and the presence of a tag can also increase the immunogenicity of some of the proteins. A tag-free protein is really more desirable for in vivo applications.
Here we can see an example of human versus bacterial expressed TNF alpha. This is a mass spec, it's comparing the molecular weight, and you can see that the authentic human TNF alpha is a 51 kilodalton mass. This is because it's a trimeric protein, whereas the E.coli TNF alpha is a third of the molecular weight.

Here you can see the same product, a HEK293 expressed TNF alpha in blue, versus an E.coli expressed TNF alpha in yellow. You can see here the higher activity of the human expressed protein on the efficiency of IL-6 production. These the in rheumatoid synoviocytes. You can see that the concentration of TNF alpha can be reduced to a fifth of what it is in the E.coli derived if you're using a HumanKine product to get the same sort of activity. This could have an effect on reducing the costs of goods in manufacturing and the quantities of cytokines that are required.
Here, onto a specific product. This is Thermostable FGF-Basic, which is stable for three days at 37 degrees, so it avoids having to feed the cells at weekends. FGF-Basic or FGF-two is a normally essential component in any cell culture. It will be an additive in complete media, and it is shown here as a standard product in the blue lines and as the thermostable product in the red line. You can see there that there are still FGF-Basic or thermostable being detected at day three.
This just gives an example of it used in real life, where you can see that the hES colonies in the first and second pictures, showing the Nanog expression in the second picture there, are maintaining pluripotency using FGF-Basic thermostable in a two-day feeding protocol. In the third and fourth pictures there, day two and day six of an IPS culture is the same sort of expansion that you would expect to see using a one-day feeding culture when using a two-day feeding culture.
Because of the product development and using stable HEK293 cell lines, it allows significant advantages in yield and predictability. That means using a HEK293 expressed protein means that there's efficient scalability there in the background to be able to move from preclinical to clinical and commercial quantities. We also have a very simple supply chain map which means that we have easy communication back the way as well.
Our products are, as standard, lyophilized in glass vials, however, we're flexible to fill and finish in different formats, for example, where glass vials are not allowed in clean rooms. We can also do custom volume requests where perhaps, as standard, we don't offer the same size as you have already been using in your process.
Here we are looking at the GMP process. As I said, this is the same as the researchers only process, there are just lots of quality check arrows on the route. Raw materials are checked coming in. Proteins are then expressed in the HEK293 cells and purified from the media. There are then an abundance of potency, purity and safety tests going on before, obviously, fill and finish and then storage. There is full traceability and quality checks on the way, to the final documentation that is released with the products. We tend to store at minus 80, but the products can be stored anywhere between minus 20 and minus 80, and as a lyophilized product, they are stable for an amount of time at ambient temperature as well.

This is our GMP facility that you saw, looking down the other way on fact, in the picture earlier on. It was opened in June last year, so it was a really quick turnaround from the acquisition to being able to have this facility opened. It's staffed by the same people that have been with the processes since it was HumanZyme, and they are really knowledgeable about the products and flipping them to GMP as well. They also set about working on SOPs and batch records straight away to be able to have these ready to manufacture to GMP, and we were able to achieve our ISO 13485 certification within nine months of the lab opening, which, particularly during lockdown, was no mean feat.
We are now able to offer GMP products using the process shown before, and this is where we fit into the advanced therapy manufacturing process. A recombinant protein is an ancillary agent, which is why the ISO 13485 is an appropriate and necessary certification to be able to user these products at GMP.

Just to into that policy in more detail, these HEK293 expressed proteins are ISO 134 certified GMP ancillary materials, so they can be used in a clinical manufacturing process. As this official statement in not so many words, it means raw materials are qualified, regular checks and qualification of clean rooms, instruments, and equipment. We have validated processes and SOPs, validated assays, well-defined standards and in-process quality checks. We've got systematic and organized training programs for personnel, documentation, and traceability as I mentioned before, and deviation and CAPAs are all recorded and acted on in a timely and efficient manner.
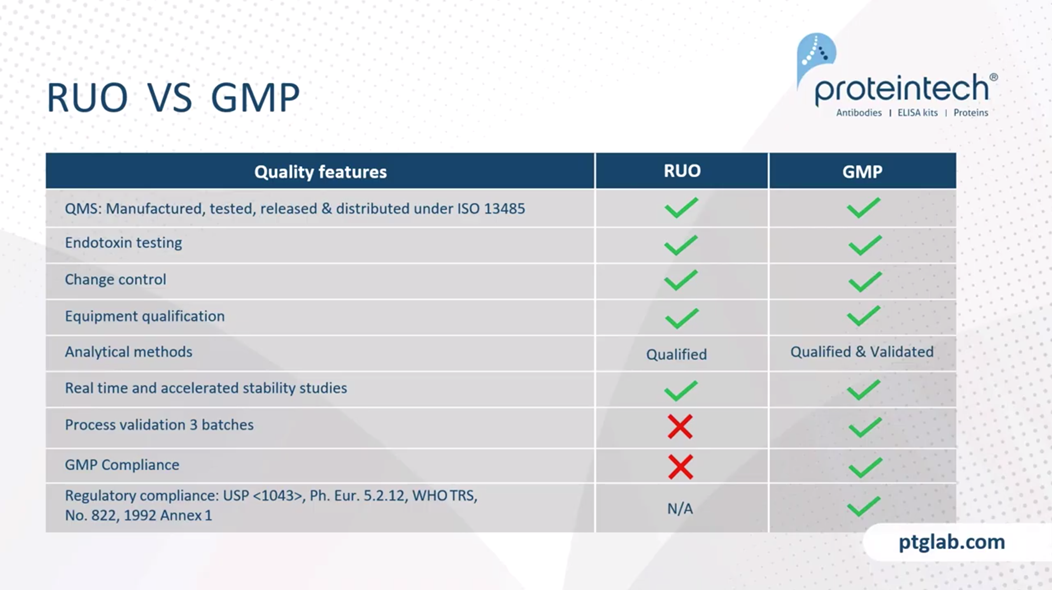
Just to look at the differences in more detail between the research and GMP version, as I said, they're manufactured in the same facility, so they can be used for the development and then the tech transfer into a GMP product under the same QMS. Endotoxin, as I've already mentioned, they're endotoxin-free. Endotoxin testing is the same for a research product and a GMP product. The change controls are there. We're using the qualified equipment. The same analytical methods, although obviously, they have to validated at a GMP level, and we have stability studies ongoing for all of products and are happy to share any stability data that may be required. Obviously, we have to do, anyone that's working in any GMP manufacturing will know we have to do the validation, three batches of each product. That means that these, the GMP products are complaint, whereas the RUO ones are not. They're in compliant with the US Pharmacopeia. In terms of ancillary materials, most territories are the same and the US Pharmacopeia seems to be gold standard through the FDA.

Just to sum up why you should give a HEK, human proteins by human cells for human applications. It makes sense. We have higher activity, stability, and these together could help reduce the cost of goods. They're endotoxin-free, tag-free, animal component-free and xeno-free, so they can be used in manufacturing of therapies for humans. There is an ease of tech transfer from RUO to GMP, and even though it might not sound like it, by putting all these things together, they're competitively priced against other expression systems that are in the marketplace. We're really happy for you to contact us for a quote to see the price differences there.
I'd like to thank you for your time and I will welcome any questions that you can type into the questions box, and I can look and see if there are any here, although you're very welcome to note down my email address, fiona@ptglab.com, and drop any questions to me directly.
Okay, so I can see we might have had some technical issues, so I apologize for any technical difficulties there may have been with sound fading as well. Please feel free to ask me for any clarifications. We will have this session recorded as well. Thank you for asking, and it will be available on the website in the next few days. We can also send it to you directly when we have that link there.
Okay, so that's all the questions I can see at the moment, so with that, I will thank you for your time this afternoon, this morning it this evening, and please feel free to get in touch with us if you have any questions on anything that I've talked about today. Thank you.




