The PD-1 / PD-L1 Pathway
PD-1 Pathway: The Immune System Stoplight
| - PD-1 Pathway Introduction | - PD-L1/CD274 Antibody |
| - Cancer Immunotherapy | - Related Products |
| - ELISA Kits | - References |
Introduction
The immune system fights off pathogens, but this defensive force can be pathogenic itself when hyperactive, resulting in autoimmune diseases such as lupus and multiple sclerosis. Consequently, the body has developed multiple mechanisms to suppress the immune system when necessary.
One method of immunosuppression is the PD-1 pathway. This pathway is activated in response to the mobilization of the immune system. The receptor PD-1 is expressed on the surface of activated lymphocytes. Similarly, its ligand, PD-L1, is expressed by antigen-presenting cells in response to cytokine signaling. When PD-L1 is bound to PD-1, downstream signaling undoes the phosphorylation events associated with activation, thereby reverting lymphocytes to an inactive state [1, 2].
| Antibody | Cat no. | Type | Applications |
| PD-1/CD279 Antibody | 18106-1-AP | Rabbit Poly | ELISA, WB, FC, IHC |
 |
Immunohistochemical analysis of paraffin-embedded human tonsillitis tissue slide using 18106-1-AP (PD-1/CD279 antibody) at dilution of 1:800 (under 10x lens) heat mediated antigen retrieved with Tris-EDTA buffer(pH9. |
Immunotherapy: A Promising Treatment for Cancer
Tumor cells take advantage of the PD-1 pathway to evade the immune system [3]. Consequently, many pharmaceutical companies have been developing drugs to inhibit PD-1 and PD-L1. In clinical trials, many patients have shown strong responses to these therapies [4-10]. For these drugs to be most effective, a high number of CD8+ T cells must already be at the tumor site, ready to be mobilized after inhibition of the PD-1 pathway [11].Recent investigations have uncovered promising methods of increasing the efficacy of PD1 inhibitors. One method is combining PD1 inhibitors with other drugs, such as HDAC inhibitors [12] and anti-CTLA4 antibodies [9]. Another method is using biomarkers to predict response to therapy [13,14]. Recent work has also suggested that GSK3 inhibitors can enhance effects of immunotherapy [15].Though these results have been encouraging, several challenges remain, including the mitigation of autoimmune effects and how to overcome drug resistance.
Current Antibody Drug Development
| Drug | Company | |
| PD-1 | SHR-1210 | Incyte |
| Nivolumab | Bristol-Myers Squibb | |
| Pembrolizumab | Merck | |
| Pidilizumab | CureTech | |
| BMS 936559 | Bristol-Myers Squibb | |
| PD-L1 | Atezolizumab | Roche |
| Durvalumab | AstraZeneca | |
| Avelumab | Pfizer/Merck | |
| MDX-1105 | Bristol-Myers Squibb | |
| CTLA-4 | Ipilimumab | Bristol-Myers Squibb |
| Tremelimumab | Pfizer/AstraZeneca |
PD-L1/CD274 Antibody
| Rabbit Polyclonal | KD/KO Validated |
| Catalog number: 17952-1-AP | 35 Publications |
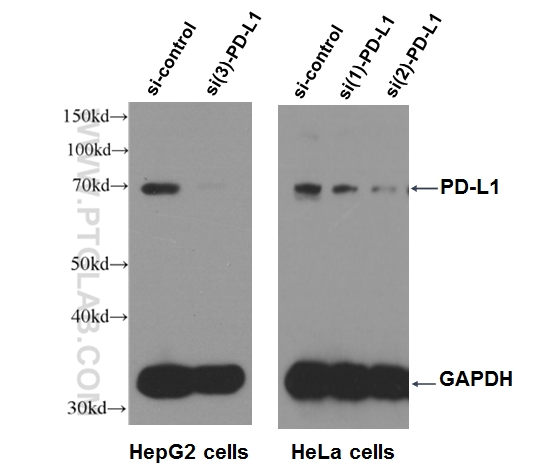 |
|
WB result of PD-L1 antibody (17952-1-AP, 1:500) with si-Control and si-PD-L1 transfected HepG2 and HeLa cells with 3 separate constructs. |
Related Products
| Antibody | Cat no. | Type | Applications |
| PD-L1/CD274 | 66248-1-Ig | Mouse mono | ELISA, WB, IHC, IF, FC |
| CD86/CTLA 4 | 13395-1-AP | Rabbit poly | ELISA, WB, FC |
| CD3 epsilon | 17617-1-AP | Rabbit poly | ELISA, WB, IHC, IP, FC |
| GSK3B | 22104-1-AP | Rabbit poly | ELISA, WB, IHC, IF, IP |
| BRAF | 20899-1-AP | Rabbit poly | ELISA, WB, IHC, IF, IP |
Loading control antibodies
| GAPDH Antibody | 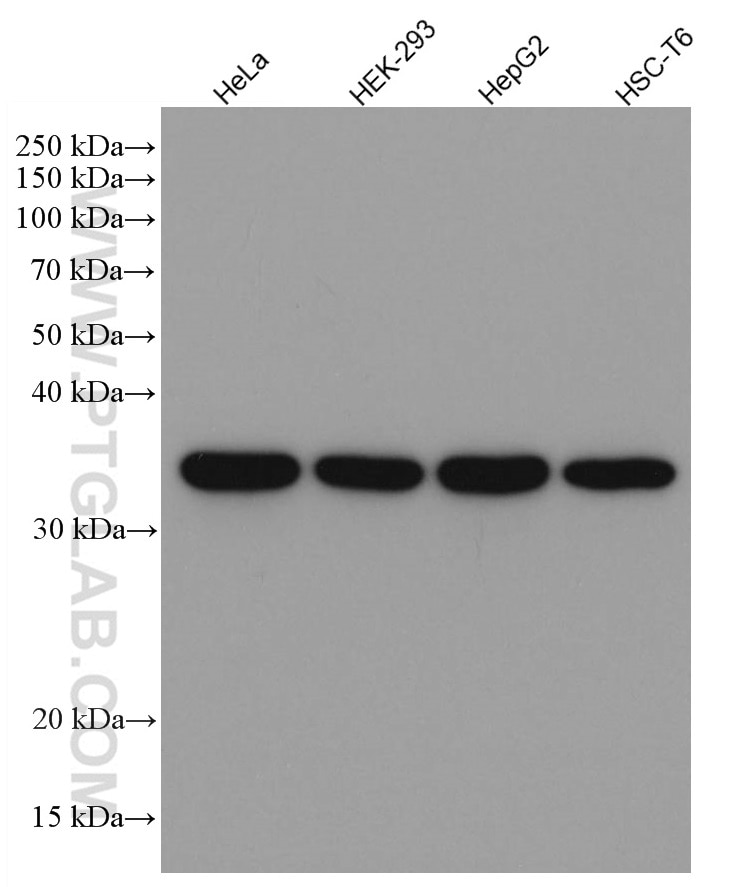 |
| Catalog no.: 60004-1-Ig | |
|
GAPDH is commonly used as a protein loading control in western blot due to its consistently high expression in most cell types. This enzyme participates in several cellular events such as glycolysis, DNA repair, and apoptosis. Proteintech monoclonal GAPDH antibodies are raised against a whole-protein antigen of human origin and have over 4,960 citations. |
| Beta Actin Antibody (KD/KO validated) | 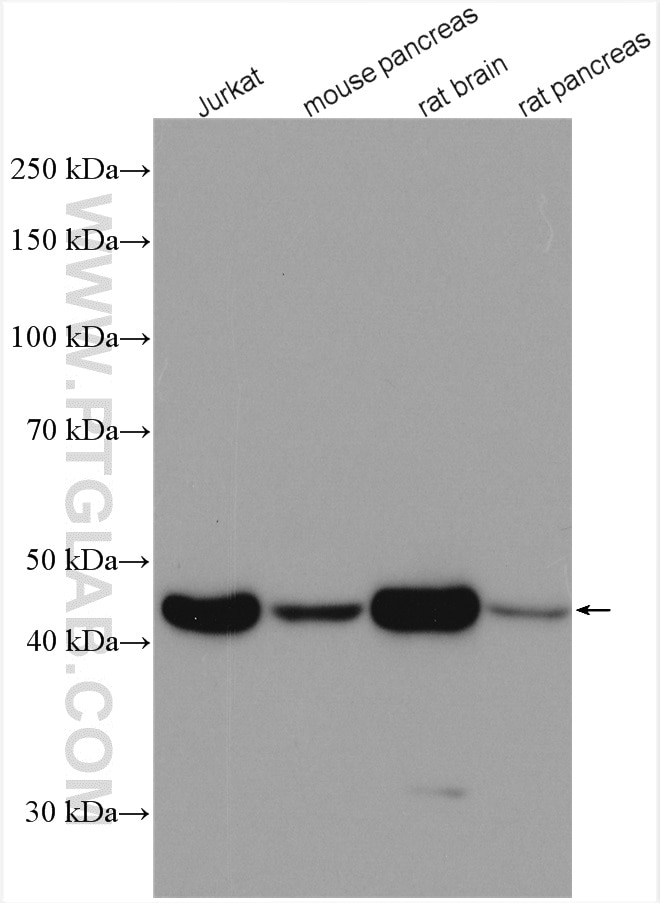 |
| Catalog no.: 66009-1-Ig | |
|
Beta-actin is usually used as a loading control due to its broad and consistent expression across all eukaryotic cell types and the fact that expression levels of this protein are not affected by most experimental treatments. 66009-1-Ig has been cited in over 2,460 publications and has wide species reactivity. |
ELISA Kits
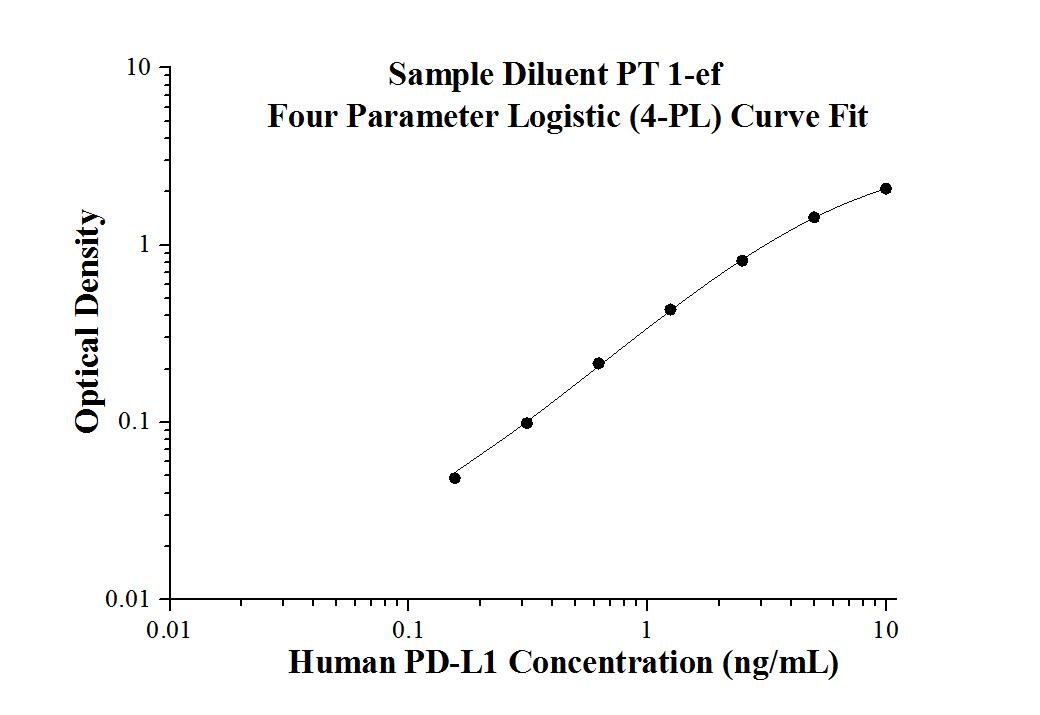
Human PD-L1 ELISA Kit |
|
Catalog number: KE00074 |
|
| KE00074 is a solid phase sandwich Enzyme Linked-Immuno-Sorbent Assay (Sandwich ELISA). The PD-L1 ELISA kit is to be used to detect and quantify protein levels of endogenous PD-L1. | |
 |
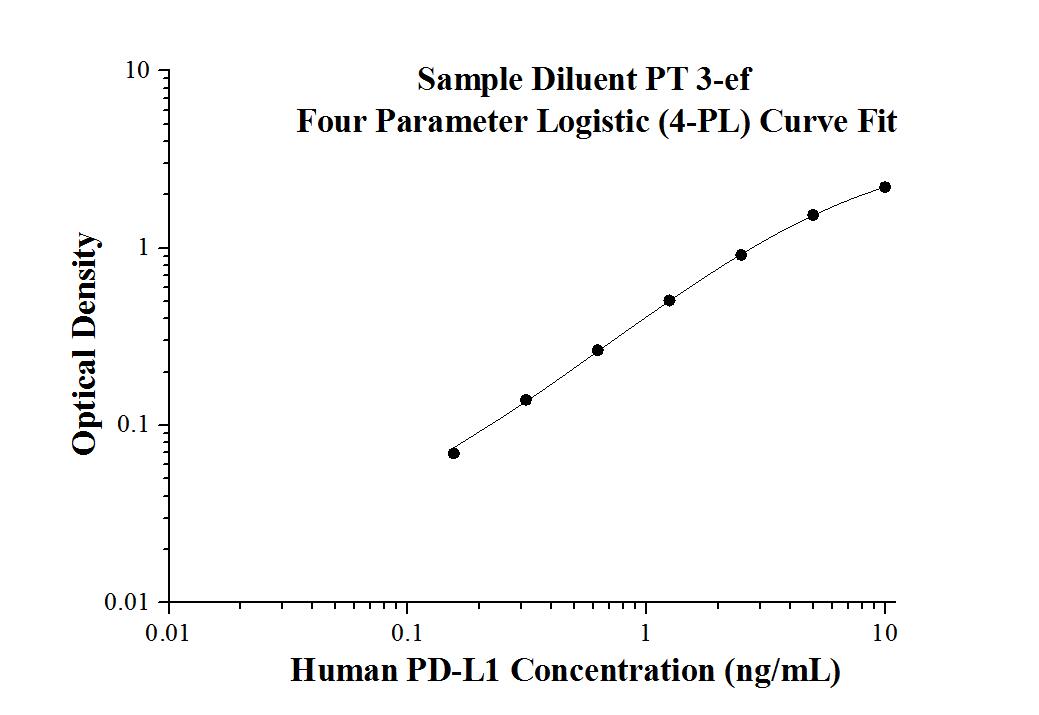 |
| Tests: | Reacted Species: |
| 1 X 96 well plate | Human |
| Sample type: | Sensitivity: |
| Serum, Plasma, Cell culture supernatant,Cell lysate | 0.04 ng/mL |
| Range: | Assay Type: |
| 0.156 ng/mL-10 ng/mL | Sandwich |
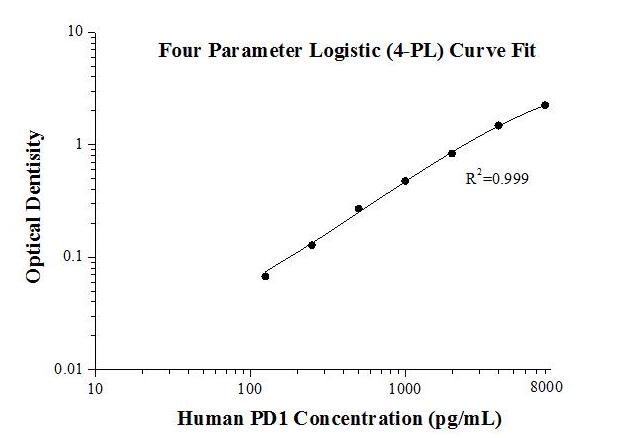
Human PD1 ELISA Kit |
|
Catalog number: KE00075 |
|
| KE00075 is a solid phase sandwich Enzyme Linked-Immuno-Sorbent Assay (Sandwich ELISA). The PD1 ELISA kit is to be used to detect and quantify protein levels of endogenous PD1. | |
 |
|
| Tests: | Reacted Species: |
| 1 X 96 well plate | Human |
| Sample type: | Sensitivity: |
| Serum, Plasma, Cell culture supernatant,Cell lysate | 43 pg/mL |
| Range: | Assay Type: |
| 125 pg/mL-8000 pg/mL | Sandwich |
References
1. Ohegbulam et al. (2015) Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 21:24-33.
2. Haanen, J. (2013) Immunotherapy of Melanoma. EJC Suppl 11:97-105.
3. Yao et al. (2013) Advances in targeting cell surface signaling molecules for immune modulation. Nat Rev Drug Discov. 12:130-146.
4. Topalian, S. et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366:2443-2454.
5. Hamid, O. et al. (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369:134-144.
6. Topalian, S. L. et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366:2443–2454
7. Brahmer, J. R. et al. (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366:2455–2465
8. Hamid, O. et al. (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369:134–144
9. Wolchok, J. D. et al. (2013) Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369:122–133
10. Topalian, S. L. et al. (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 32:1020–1030
11. Tumeh, P. et al. (2014) PD-1 blockade induces responses by inhibiting adaptive immune response. Nature. 515:568-71.
12. Woods, D. et al. (2015) HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol Res 12:1375-85.
13. Chakravarti, N., & Prieto, V. G. (2015). Predictive factors of activity of anti-programmed death-1/programmed death ligand-1 drugs: immunohistochemistry analysis. Translational Lung Cancer Research, 4:743–751.
14. Barak, V. et al. (2015) Assessing response to new treatments and prognosis in melanoma patients, by the biomarker S-100B. Anticancer Res. 35:6755-60.
15. Taylor, A. et al. (2014) Glycogen synthase kinase 3 inactivation drives T-bet-mediated downregulation of co-receptor PD-1 to enhance CD8+ cytolytic T cell responses. Immunity 44:274-86.




