Cancer and the Immune System
The interaction between cancer and immune cells is a key area of research and an expanding therapeutic approach
Why is the immune system important for cancer research?
Immune evasion and chronic inflammation are both hallmarks of cancer as defined by Hanahan and Weinberg. Solid tumours are composed of cancer cells, extracellular matrix (ECM) and accessory cells such as immune and endothelial cells. Cancer cells can modify the immune cells in their microenvironment not only to evade attack by immune cells but also help support tumour growth. This is observed with Tumour-Associated Macrophages (TAMs), Tumour-Associated Neutrophils (TANs), modified T cells and others.
The recruitment of modified immune cells also leads to tumour-associated chronic inflammation which itself contributes to further genomic instability, epigenetic modification and anti-apoptotic pathways, generating a vicious cycle of tumour progression.
Disseminated tumour cells also evade immune attack when they are travelling through the blood or lymphatic system. As tumour cells colonise a distant organ during metastasis, they modulate the organ’s immune microenvironment to allow survival and colonisation.
Immunotherapy as a treatment against cancer
The importance of cancer cells and their immune microenvironment is increasingly characterised allowing immunotherapies to exponentially develop. These immunotherapies offer a more targeted approach than the current “gold standard” for cancer treatments: surgical resection, chemotherapy and radiotherapy. These treatments are accompanied with numerous side effects and are not suitable for all cancer types. Specifically, certain cancer types are extremely rare and resistant to standard therapy and therefore require an alternative therapeutic strategy, some of which are listed below.
- Monoclonal antibodies – Recombinant antibodies which target a cancer-specific antigen, signalling to the immune system which cancer cells to kill. These are particularly useful in cancers with well-characterised markers. For example, herceptin® (Trastuzumab) is a monoclonal antibody therapy targeting the HER2 receptor and has had significant success against HER2+ breast cancer subtypes. Avastin® (Bevacizumab) is an anti-angiogenic monoclonal antibody therapy targeting VEGF and is used as an anti-metastatic agent in combination with chemotherapy in colorectal cancer.
- CAR T therapy - This new and exciting approach to immunotherapy has had extremely positive clinical response in patients with systemic cancers such as leukaemia and lymphomas, who cannot benefit from standard treatments including surgery or radiotherapy. This therapy harnesses T cells’ natural cytotoxic abilities and targets them against cancer cells. A patient’s T cells are isolated and transfected to express a cancer-targeting Chimeric Antigen Receptor (CAR). These cells then undergo clonal expansion and are transfused back into the patient to enter the circulation and target the cancerous cells. (cGMP grade cytokines and growth factors).
- Immune checkpoint inhibitors – These drugs target inhibitory receptors expressed on immune and cancer cells to stimulate an anti-tumour immune response. (PD1-PDL1 pathway)
- Cancer Vaccines – similarly to a standard vaccine, these introduce a cancer-specific antigen into the patient to stimulate an immune response against the cancer.
How to study the immune system in the context of cancer research?
-
In the primary tumour – immune cell infiltration can either promote or prevent tumour growth depending on the cancer and the type of immune cell present in the tumour. IHC is one way to assess the level of infiltration. Our IHCeasy kits for targets such as CD3, Cd11b, F4/80 enable easy identification of the location of specific immune cells (e.g. macrophages, T cells, neutrophils) within the tumour. Flow cytometry, which offers a more quantitative approach, can also be used to assess immune cell infiltration in the primary tumour.
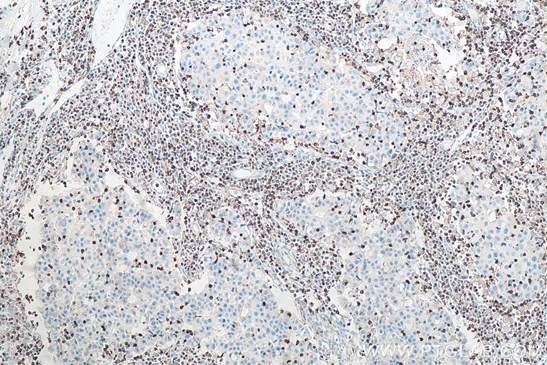 |
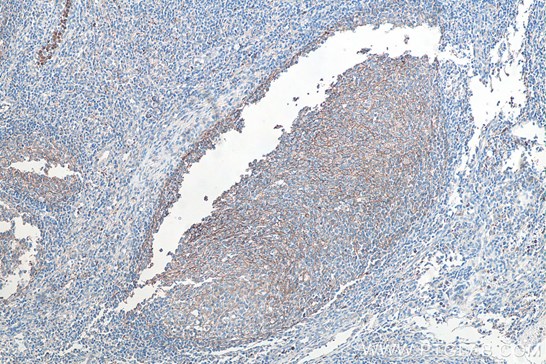 |
| Fig1: Immunohistochemical analysis of paraffin-embedded human breast cancer tissue slide using KHC0013 (CD3 IHCEasy Kit) (T cell marker) | Immunohistochemical analysis of paraffin-embedded human tonsillitis tissue slide using KHC0027 (CD11B IHCEasy kit) (macrophage marker) |
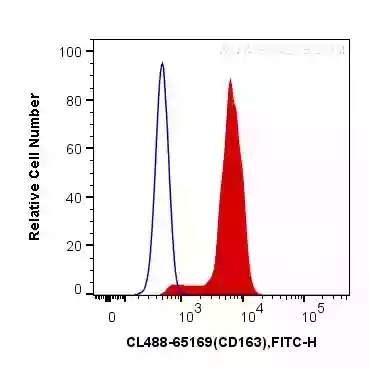
Fig.2: 1X10^6 human peripheral blood monocytes were surface stained with 5 ul CoraLite®488 Anti-Human CD163 – a tumour-associated macrophage (TAM) marker (CL488-65169, Clone:GHI/61) (red), or Mouse IgG1 Isotype Control (CL488-66360, Clone: T1F8D3F10) (blue). Cells were not fixed.
-
Systemically - Immune cells in circulation can be analysed at different stages of cancer progression via blood sampling. Flow cytometry can give a snapshot of the immune profile in the bloodstream at different stages of cancer progression.
-
At metastatic sites - The immune profile of organs at different stages of cancer (with/without metastases present) can be investigated using Flow Cytometry, IHC, IF or Western Blotting. Disseminated tumour cells interact with and modulate distal organs during metastasis, including modifying their immune landscape to a more favourable environment for their survival. Understanding the changes in these target organs could help prevent tumour cell survival and colonisation at these sites.
Table 1: Explore our antibodies for immune markers:
|
Ly6C |
||
|
|
Check out our Flow Cytometry page to design a multi-colour panel and explore the range of fluorescent dyes available.
Blog written by Lucie Reboud, 4th year PhD Student at the University of Manchester and Science Marketing Intern for Proteintech.
References:
Gonzalez, H., Hagerling, C., & Werb, Z. (2018). Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes & development, 32(19-20), 1267-1284.
Sterner, R. C., & Sterner, R. M. (2021). CAR-T cell therapy: current limitations and potential strategies. Blood cancer journal, 11(4), 1-11.
Finak, G., Langweiler, M., Jaimes, M., Malek, M., Taghiyar, J., Korin, Y., ... & McCoy, J. P. (2016). Standardizing flow cytometry immunophenotyping analysis from the human immunophenotyping consortium. Scientific reports, 6(1), 1-11.
Hiam-Galvez, K. J., Allen, B. M., & Spitzer, M. H. (2021). Systemic immunity in cancer. Nature reviews cancer, 21(6), 345-359.





