Cancer and Metastasis
Metastasis is the leading cause of cancer-related deaths
What is metastasis?
Metastasis is the multi-stage process by which some cancer cells leave the primary tumor and colonize a distant organ to form secondary tumors. This represents a significant clinical burden as metastasis is the leading cause of cancer deaths and prevents the use of certain treatment options including surgery and radiotherapy. Understanding and inhibiting the different stages of metastasis is an active area of cancer research.
The stages of metastasis
The metastatic cascade comprises a series of steps that cancer cells undergo, starting with detachment from the primary tumor to the formation of secondary tumors. Each step represents an obstacle for cancer cells to overcome and serves as a selection process.
Steps in metastasis:
- Local invasion and migration
- Intravasation into bloodstream
- Dissemination via blood or lymphatic circulation
- Adhesion to endothelial walls and extravasation
- Survival and colonization at a distant site
- Secondary tumor formation

Fig. 1: Diagram of the metastatic cascade and the markers associated with each step
How to study the different stages of metastasis?
Understanding the steps that lead to distal organ metastasis will allow inhibition at multiple stages and a higher chance of prevention.
We have put together some useful resources covering the topics below:
- EMT (epithelial-mesenchymal transition) of cells in the primary tumor – cells with a more mesenchymal (and less epithelial) phenotype usually have a higher propensity to migrate and metastasize. See: Useful markers of EMT
- Secretion of ECM (extracellular matrix) remodeling enzymes by cancer cells. See: MMP ELISA and IHC Kits
- Markers of angiogenesis needed to invade local tissue. See: VEGF as marker of angiogenesis
- Quantification of circulating tumor cells (CTCs) by flow cytometry detection (See Table 1 below for markers)
- Colonization and micro-metastases formation in secondary organs – general markers of proliferation (Ki67), cell line-specific markers, MET markers.
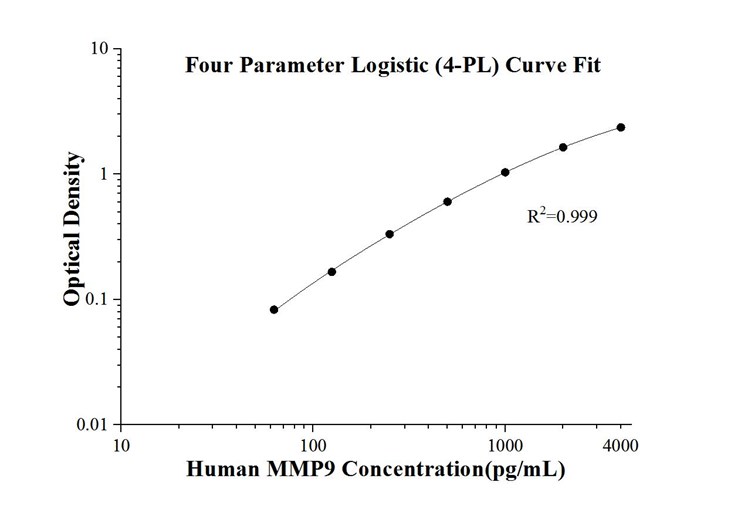
Fig.2: Human MMP9 solid phase sandwich ELISA Kit KE00164 Standard Curve used for detection of secreted MMP9 levels from human serum or cell culture supernatant.
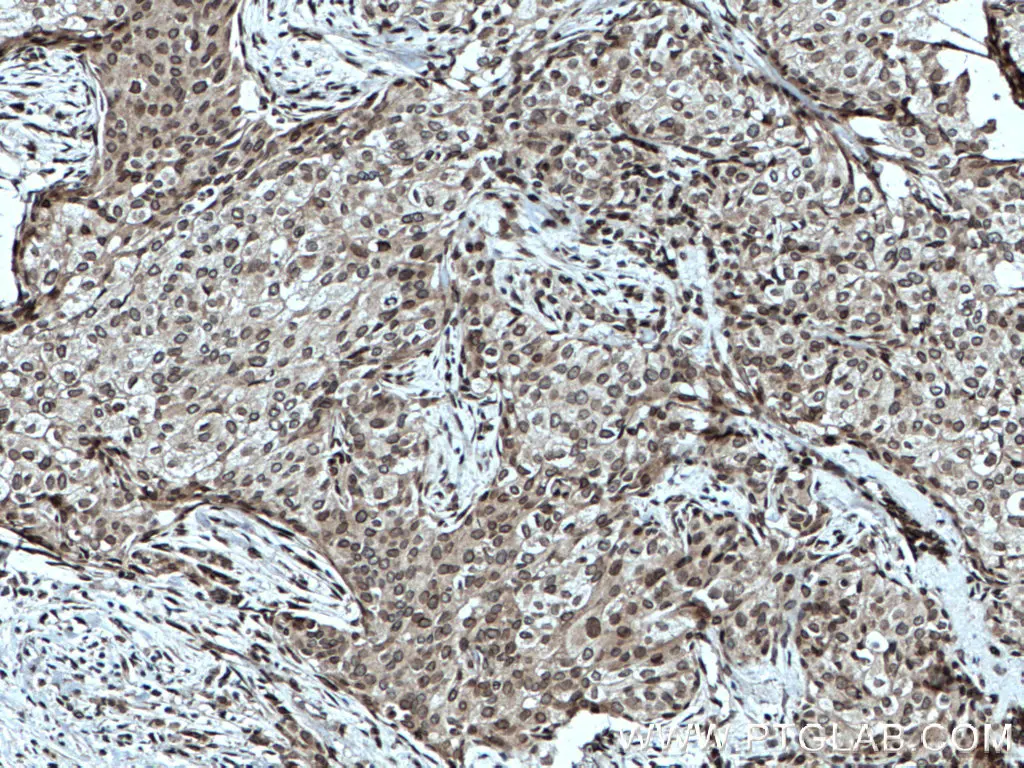
Fig3: Immunohistochemical analysis of paraffin-embedded human breast cancer tissue slide using 25465-1-AP (TWIST1-specific antibody) at dilution of 1:200 (under 10x lens. Heat-mediated antigen retrieval with Tris-EDTA buffer (pH9.0)
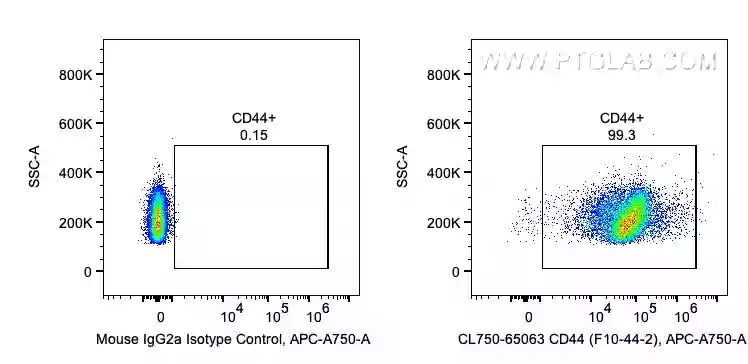
Fig 4: 1X10^6 human PBMCs were surface stained with 5 ul CoraLite®750 Anti-Human CD44 (CL750-65063, Clone: F10-44-2) . CoralLite has a range of colors to suit different panels (e.g., 488nm CL488-65117, 555nm CL555-65117, 568nm CL568-65117).
Table 1: Markers for detection or isolation of CTCs using Flow Cytometry:
Marker |
Specificity |
|
Epithelial |
|
|
Epithelial |
|
|
Mesenchymal |
|
|
CSC (Cancer Stem Cell) marker Metastatic Marker |
|
|
Cancer Stem Cell Breast Cancer |
|
|
CSC Metastasis Many Cancer Types |
|
|
Colorectal Cancer Prostate Cancer Breast Cancer Use in combination with other markers |
|
|
CSC Adhesion Molecule |
|
|
Mesenchymal Melanoma Breast Cancer Use in combination with other markers as is also an endothelial marker |
|
|
Negative Control to exclude immune/HSC lineage cells from blood or tissue |
|
|
For GFP tagged cancer cells |
Find more CTC markers for flow cytometry by cancer type here
Cancer cells can also be detected using IHC – See IHCEasy Kits for Cancer specific markers
Blog written by Lucie Reboud, 4th year PhD Student at the University of Manchester and Science Marketing Intern for Proteintech.
References
Mina, L. A., & Sledge, G. W. (2011). Rethinking the metastatic cascade as a therapeutic target. Nature reviews Clinical oncology, 8(6), 325-332.
Dianat-Moghadam, H., Azizi, M., Eslami-S, Z., Cortés-Hernández, L. E., Heidarifard, M., Nouri, M., & Alix-Panabières, C. (2020). The role of circulating tumor cells in the metastatic cascade: biology, technical challenges, and clinical relevance. Cancers, 12(4), 867.
Aiello, N. M., & Kang, Y. (2019). Context-dependent EMT programs in cancer metastasis. Journal of Experimental Medicine, 216(5), 1016-1026.
Chitty, J. L., Filipe, E. C., Lucas, M. C., Herrmann, D., Cox, T. R., & Timpson, P. (2018). Recent advances in understanding the complexities of metastasis. F1000Research, 7.
Related Content
Cancer stem cells as a key to cure cancer
Molecular markers for liver cancer

Support




